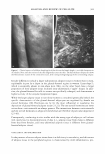
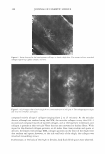
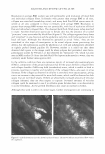
2005 ANNUAL SCIENTIFIC SEMINAR IR array ckttector ! Mkrosc:ope � I Spectrometer I - array ot 4096 complete IR spectra - 1: 1 mapping of 7x7 µm sample spot lo deteclor element Figure 1: Schematic of R imaging m1aoscopewtth6'x&• element anayoetecro, The Ii,.• array s,.tems bulld up a atray of lla1'1 by rnow,g gmple stage - .... Flgulw Z: The opllcal image ol a lamellar lipid sunscreen 111m and the spectrum fntn one elemenl in lhe collected aray ol data. The specsun smws 1he un,que c-;a,o peak ot lhe UV absorbing molecues. The IR spectroscopic Im• ,,_, the rotio ol the UV obsorbin1 sun,c,- loctocryl"""I intensity 111alnst that ol the lip d cti.in, (2218/2852 cm·•]. Mote, the dlfleN!nce in Intensity ocron this lmote does not represenl dlfleence, in film thickneH but rather the mole(ular distribution ol sunscree-n tn the formulMion. REFERENCES 477 [l] R. Mendelsohn, M.E. Rerek, D.J. Moore, "Infrared Spectroscopy and Microscopic Imaging of Stratum Comeum Models and Skin," Physical Chemistry Chemical Physics, 2, 4651-4657 (2000) [2] R. Mendelsohn, H-C.Chen, M.E. Rerek, D.J. Moore, "Infrared microspectroscopic imaging maps the spatial distribution of exogenous molecules in skin," J. Biomed. Opt.8, 185-190 (2003) [3] C. Xiao, D.J. Moore, M.E. Rerek, C.R. Flach, R. Mendelsohn, "Feasibility of Tracking Phospholipid Permeation into Skin Using Infrared and Raman Microscopic Imaging," J.lnvest.Dermatology, 124 (2005) [4] L. H. Kidder, V. F. Kalasinsky, V. F. Luke, I. W. Levin, and E. N. Lewis, "Visualization of silicone gel in human breast tissue using new infrared imaging spectroscopy," Nat. Med. 3, 235-237 (1997) [5] E. N. Lewis, A. M. Gorbach. C. Marcott, and I. W. Levin, "High fidelity Fourier transform infrared spectroscopic imaging of primate brain tissue,'' Appl. Spectrosc. 50, 263-269 ( 1996) [6] R. Mendelsohn, E. P. Paschalis, and A. L. Boskey, "Infrared spectroscopy, microscopy, and microscopic imaging of mineralizing tissues: spectra-structure correlations from human illiac crest biopsies," J. Biomed. Opt. 4, 14-2 I ( 1998)
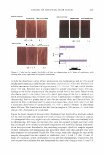
478 JOURNAL OF COSMETIC SCIENCE NOVEL SILICONE VESICLES FOR DELIVERY OF ACTIVES IN PERSONAL (ARE Shaow B. Lin1, Ph.D., Stephanie Postiaux2 and Joanna Newton2, Ph.D. 1 Delivery Technology, Science Ei Technology, Specialty Chemicals Business, Dow Corning Corp., Midland, MI 'Application Development, Life Science Innovation Team, Dow Corning Europe, Seneffe, Belgium Introduction: Stabilization and efficacy enhancement of lipophilic actives in aqueous cosmetic products are important formulation needs in the personal care industry. Traditional encapsulating technologies convert the actives into capsules or particles of micron-sizes or larger, providing mainly the stabilization function with little efficacy benefit. There is a growing interest for a nano- to submicron-sized delivery system that provides both benefits. In response to these unmet needs, we successfully developed a novel method for preparing "assembly-required" silicone vesicles from hydrophobic silicone polyethers. Vesicles were made and shown stable from hydrophobic PEG-12 dimethicones (rake-type silicone polyethers) using this patent-pending process [1,2]. The relation between PEG-12 dimethicone architecture and vesicle-forming capability was also shown. These vesicles are fundamentally different from the self-assembly vesicles from hydrophilic PEG-12 dimethicones known in prior art [3,4]. This paper presents a new assembly-required silicone vesicle derived from an (AB) n silicone polyether block copolymer. The utility of this polymeric silicone vesicle as a topical delivery system for lipophilic personal care ingredients is discussed. Materials and Methods: Dimethicone PEG-12 copolymers (INCi name to be proposed) are (AB) n alternating block copolymers of PEG-12 polyether blocks (represented by A, where 12 is the average number of oxyethylene units in PEG block) and dimethicone blocks (represented by B), with n representing the number of AB repeats in the copol ym er. Dimethicone PEG-12 copolymers with various dimethicone block lengths, illustrated in Figure 1, were made at Dow Corning for this study. These dimethicone PEG-12 copolymers are hydrophobic, with poor solubility in water. [-� [� [- - PEG-12 block (A) � Dimethicone block (B) Figure 1. Dimethicone PEG-12 copol ym ers with different dimethicone block lengths. Cryogenic transmission electron microscopy (Cryo-TEM) was used to visualize the vesicles. This is done by freeze drying the vesicles at cryogenic temperature, then analyzing the dried specimens at cryogenic temperature. Vesicle size and distribution were determined using a Nanotrac particle analyzer. Formulation development was carried out to determine formulation latitude and compatibility with common cosmetic ingredients. Cryo-TEM was used to verify the integrity of vesicles in formulations, and HPLC assays to quantify the amount of vitamin in vesicles. Results and Discussion: The dimethicone PEG-12 copolymers prepared in this study were hydrophobic and exhibited poor to no solubility in water. We successfully prepared submicron-size vesicles from (AB)0 dimethicone PEG-12 copolymers of selected dimethicone block length, using a Dow Corning proprietary process. Water-insoluble dimethicone PEG-12 polymeric molecules arranged themselves into bilayers and vesicles in the presence of a specific medium. These structures were then kinetically locked to achieve stability. Figure 2 illustrates how the polymeric dimethicone PEG- 12 molecules transform themselves into orderly bilayers and vesicles.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)