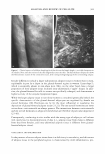
400 JOURNAL OF COSMETIC SCIENCE Table I Chemical Shifts for 13C-NMR Spectra of Inulin and Levan Produced by Zymomonas mobilis* C-1 C-2 C-3 C-4 C-5 C-6 Reported in reference 22 Inulin ([) 2➔ l) 60.9 103.3 77.0 74.3 81.1 62.2 Levan ([) 2➔6) 5 9.9 104.2 76.3 75.2 80.3 63.4 Measured Levan ([) 2➔6) 5 9.9 104.2 76.3 75.3 80.4 63.5 * Chemical shifts (o, ppm) were calibrated and reported from TMS (tetramethylsilane) as internal standard. Samples were prepared in D20 (about 10 mg/0.5 ml of D 2 0). self-forming levan nanoparticles was determined via the laser-light-scattering method on an ELS-8000 particle-size analyzer (Otsuka Electronics, Japan) in water and 20% aque ous ethanol (by weight) (Figure 1). The mean particle sizes of these complexes were 224.3 nm and 251.8 nm, respectively. The results of transmission electron microscopy were concordant with the results of the dynamic light scattering described above, which indicated self-formed nanoparticles and their agglomerates existing in a size range of several hundred nanometers (Figure 2). Demel et al. (28) reported that fructans strongly interact with lipid model membranes, due to its high solubility, which would indicate that hydroxyl groups are primarily available for interactions with surrounding water molecules as well as membrane phos pholipid head groups. Vereyken et al. (29) reported that fructans exhibited much stron ger effects on different lipid systems than did other polysaccharides. This phenomenon was attributed to fructans' hydrophobic properties, which in turn are due to the addi tional CH 2 group in furanosides, as compared with pyranosides such as dextran. Con sidering the above, levan not only exhibits hydrophilicity, but also hydrophobicity, like a surfactant. This quality helps to explain why levan tends to form nanoparticles in water. ST ABILITY TEST IN ETHANOL Aqueous levan solution (5 % on a solid basis) was then mixed with ethanol at different SIZE ,,, DISTRIBUTION c weight 10 8 6 2 0 . " . . . . . . . . . . " 124 177 252 (a) AUT02 9.4E+01 t:ln(D) (11nm) 360 (0nm) SIZE DISTRIBUTION ( weight ) AUT02 (%) 10 92E+01 8 .. ..L 6 dln(D) 4 (1mm) 2 ... 90 125 179 256 367 (OMI) (b) Figure 1. The weight average particle size distribution of levan: (a) 5% levan in water (b) 5% levan in 20 wt% aqueous ethanol.
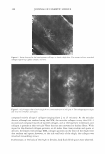
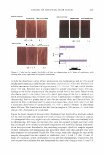
COSMECEUTICAL PROPER TIES OF LEV AN 401 Figure 2. TEM micrograph of levan showing self-formed nanoparticles. concentrations, ranging from 10 wt% to 50 wt%. Turbidity and precipitation were observed in order to determine the storage stability at room temperature, 5°C, and 40°C for a total of 45 days. As a result of these observations, we determined the solubility of the levan solution in ethanol to be so excellent that we were completely unable to observe any precipitation, and the solution was found not to be turbid (Table II). MEASUREMENT OF MOISTURIZING EFFECT To evaluate the moisturizing effects exerted by levan, we measured transepidermal water loss (TEWL) and moisture content with a Corneometer CM825. The TEWL results of the levan applied to the skin were then compared with the results of 0.2% hyaluronic acid and distilled water (Figure 3). Our statistical analysis of this data revealed a significant decrease in water loss on the skin as the result of the application of levan and hyaluronic acid, as opposed to when distilled water was applied. We then measured the moisture content of 0.2% levan and 0.2% hyaluronic acid with the Corneometer CM825 Table II Stability Test of Levan in Aqueous Ethanol 1 Ethanol (%)2 10 20 30 40 50 7 stable stable stable stable stable 15 stable stable stable stable stable 1 Levan was used at a concentration of 5 % on a solid basis. 2 Ethanol(%) was expressed by weight of ethanol in water. 3 Kept at room temperature, 5°, and 40°C for 45 days. Period (days)3 30 45 stable stable stable stable stable stable stable stable stable stable
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)