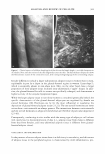
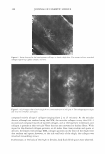
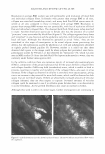
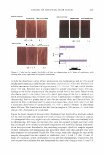
2005 ANNUAL SCIENTIFIC SEMINAR 451 This combination solubilizes on the surface of the oil droplets making them more water compatible. Additional surfactant mixture, then coalesces as multiple bi-layers separated by water phase "layers". These bi-layer structures are the surfactant hydrate structures that prevent the close approach and coalescence of the oil droplets. (Fig.1) Figure 1 From Eccleston, GM , Multiple-Phase oil-In-water emulsions J. Soc. Cosmet. Chem 41,1�22 (1990) In order to analyze the structures that form and stabilize the emulsions, I have chosen to compare formulas that contain 15% mineral oil, 4% cetearyl alcohol and are made with either an anionic, cationic, nonionic or a polymeric surfactant. The structures that form can be liquid crystals, gel phase (as in Fig.1) or gelled (structured) water phase in the case of the polymeric emulsifiers. Analysis is accomplished through microscopic evaluation of the emulsions. Particle size analysis can be used to estimate stability, but, more importantly, liquid crystals and gel phase regions (i.e. surfactant hydrate structures) can be identified by looking at the emulsions through a system of polarizing filters. The liquid crystalline and gel phase materials reorient the angle of polarization of the incident polarized light allowing some light to be seen through a polarizing filter whose axis is crossed at 90 ° to the axis of the incident polarized light. Analysis of polymeric surfactant based emulsions shows that these emulsions can also contain regions that interact with polarized light, although this interaction may be due to structures formed by the fatty alcohols contained in the formulas. Storing these formulas at elevated temperatures affects the amount and nature of the surfactant hydrate structures that form in the emulsions and these changes can be used to estimate the application feel and the stability of emulsions.
452 JOURNAL OF COSMETIC SCIENCE AMPHOTERIC SURFACTANTS Euen Gunn Rhodia Amphoteric surfactants have been used by the cosmetic industry for now more than forty years. Amphoteric surfactants based on carboxymethylation of coconut irnidazolines or coconut amidoarnines are now well established as extremely mild surfactants (American College of toxicology, 1990). They are widely used in mild, tear free shampoos and sensitive skin cleansers due to their favorable surfactant properties, low irritation profile, and irritation mollifying properties known as detoxification (A.LL Hunting, 1985 G. Panzer, 1980). But over the past few years due to a reRewed interest in the fundamental properties of amphoteric surfactants, extensive research effort now shows that there is more functional attributes besides their intrinsic properties. Byproducts which used to be considered as impurities have been shown to play an extensive role in controlling foaming and rheological behaviour of cosmetic formulations. The product composition can then be tuned to provide tailorrnade performances. But this is in the field of surfactant I surfactant interactions and their interactions with polymers that the most existing insights have been discovered. In formulations containing conditioning polymers, mostly cationic polymers, what were previously identified as incompatibility areas in the phase diagram can now be turned into a powerful way for optimizing the delivery of a given attribute. Precipitation of the surfactant / polymer complex can be controlled through several features such as chemical nature of the surfactants, electrolyte content, nature of the counterions, electrostatic balance of the polymer, hydrophobicity of the polymer, ... This paper will present key features of amphoteric surfactants and how controlled interactions with cationic polymers can be tuned into a powerful delivery system.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)