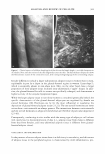
2005 ANNUAL SCIENTIFIC SEMINAR proceed from the superficially residing polyquat-10 to the cortex resident CET AB and panthenol, there is a distinct shift in the slope towards more positive values (The rather low value for k at 60°C cannot be accounted for at the present). Though at lower temperatures (40°C) the three actives on hair have about the � --+- Control --- Water -•- CETAB --T--- Polyquat-10 ♦ Panthenol 80 oc 60 40 1.85 �-'-------I---------+-� 1.80 1.75 1.70 1.65 - 1.60 - 1.55 - 1.50 -+-----.----�--�---�--' 0.0028 0.0029 0.0030 0.0031 1fT 0.0032 Fig. 1 Arrhenius plot for the stress relaxation kinetics in variously treated hair fibers same effect on stress relaxation kinetics, there is divergence as the temperature is elevated, especially after 60°C. It appears as if the presence of either CET AB or panthenol in the cortical region impedes stress relaxation. More specific experiments are needed to understand how these compounds are affecting the stress relaxation mechanism. One hypothesis could be that these actives, through some weak bonding (either chemical or van der Waals) stiffen the cortex of the bleached hair slowing down the relaxation process. The enhanced temperature could be playing a role in promoting such bonding interactions between the host the guest materials. This hypothesis has to be reconciled with the behavior of fibers that were just treated with deionized water. It is seen from Fig. 1 that water treated fibers behave almost similar to those treated with CET AB. It is likely that this is due to the leaching of degraded keratins which would interfere with hydrogen bonding or salt link formation in the cortex (especially in the matrix). Subject to confinnation by further experiments, it is clear that TMA results are sensitive to the location of the actives that hair tresses are treated with, showing a gradation in the Arrhenius plot as actives are sorted from the purely surface resident one through those diffusing to the cortex. This could be used as an exploratory tool for studying potential actives for hair. 469
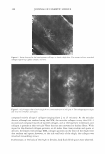
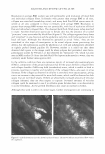
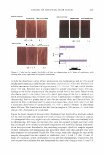
470 JOURNAL OF COSMETIC SCIENCE THE ANTI-ANGIOGENIC AND ANTI-INFLAMMATORY ACTION OF MENTHOL PROPYLENEGLYCOL CARBONATE (MPC) IN EXPERIMENTAL MODELS Johnathan R. Matias, Ph.D. Poseidon Sciences Group, 122 East 42nd Street, Suite 1700, New York, NY 10168 INTRODUCTION Active research in the use of naturally-occurring biochemicals for application as topical medication is motivated in part, by growing public concern on the possible health risks associated with products that contain new synthetic active agents. Skin inflammation as a result of dermatitis, psoriasis and eczema remain among the major applications of novel anti-inflammatory research efforts. Menthol has been in human use for almost a century in a wide range of applications including treatment for inflammation of the skin. However, this inherent anti-inflammatory effect is limited by its low biological activity. By studying structure-activity relationships, specific menthol analogues possessing strong biological activities suitable for practical use were identified. The addition of a propyleneglycol carbonate moiety on the menthol structure results in a cooling agent known by its common name, menthol propyleneglycol carbonate (MPC), a compound listed as a FEMA GRAS compound since 1999. This paper describes the anti-angiogenic and anti-inflammatory effect of MPC in experimental models. MATERIALS AND METHODS The antiangiogenic effect of MPC was studied by culturing aortic explants in three-dimensional matrix gels according to the procedure of Kruger and Figg (I). Thoracic aortas were excised from 8- week-old male Sprague Dawley rats and the fibroadipose tissue removed. The aortas were sectioned into I-mm cross-sections, rinsed with Human Endothelial-SFM (GIBCO), placed on the Matrigel-coated wells, covered with an additional 50 µl Matrigel, and allowed to gel for more than 30 min at 37°C, 5% CO2, All the rings were cultured in Human Endothelial-SFM (GIBCO), supplemented with 200µ1/ml of ECGS (Endothelial Cell Growth Supplement, Sigma) as an angiogenesis inducer. MPC, diluted with ethanol, was added to the culture medium at final concentrations of I µM, IOµM, and I00µM. Ethanol alone (1%) was added to the culture medium as vehicle control. The area of angiogenic sprouting was calculated using Image-Pro Plus software (Media Cybernetics). Microvessel densities are reported in square pixels. All assays were performed using 5 aortic rings per sample and were photographed on day 10. The anti-inflammatory effect of MPC was evaluated using tetradecanoylphorbol ester (TPA) induced edema in the mouse ear. The mouse ear edema model is a standard animal test procedure to document the anti-inflammatory effect of an agent. In this test, edema was induced in mice through the topical application of 10µ1 of TPA in acetone (2.5µg/ear) to both the inner and outer surface of one ear of each mouse. Each test compound, diluted with acetone to a concentration of 10%, was applied topically to the inflamed mouse ear immediately after TPA application, so as to deliver 2.5 mg/ear. The reference drug, indomethacin (0.5mg/ear), was administered as a positive control. The thickness of each ear was measured before treatment and 4 hours after induction of inflammation, using a micrometer (Mitutoyo Co.). Anti-inflammatory effect was expressed as the reduction in ear thickness with respect to the control group. RESULTS The data in Table I and Figure I show that MPC exerted significant anti-angiogenic activity in a dose-dependent manner. When MPC was added to cultured rat aortic explants, there was a dose dependent inhibition of the formation of new microvessels. This inhibition of microvessel "sprouting" was not due to cytotoxic effects, since Ha-CaT and HUYE (human umbilical vein endothelial cells) cell lines were not affected by the same doses used in the angiogenesis assay. The inhibitory effect on the inflammatory response was tested by the topical application of MPC (referred to in the graph as HR-008) at 2% and 10% on the mouse ear. The results of the experiment are presented in Figure 2. The study
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)