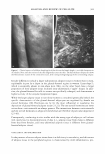
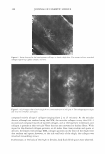
404 JOURNAL OF COSMETIC SCIENCE 110 .....-----------------------, - 100 � ·s: :1 □ I I I □ J (a) (b) (c) (d) Concentration (w/v°/4) Figure 7. Cell proliferation effect of levan in 3-D artificial skin: (a) control (b) 0.05% SDS (c) 0.05% SDS + 0.01 mg/ml of levan (d) 0.05% SDS + 0.05 mg/ml of levan. The data are expressed as mean values (± standard deviations) of four experiments. ANTI-INFLAMMATION TEST WITH 3-DIMENSIONAL ARTIFICIAL SKIN Interleukin-la. (IL-lu) is known to function as a proinflammatory mediator during intercellular signal transport, and has also been shown to induce the proliferation of some cells, including osteoblasts, m·onocytes, macrophages, keratinocytes, hepatocytes, and fibroblasts, via stimuli such as inflammation or infection (30). IL-lu is also known to stimulate the increase of arachidonic acid lipoxygenase metabolites, including leukotri ene B4, 5-, 12-, and 15-HETE, and also functions as a potential inducer of reepitheli alization in wounded skin (31). Therefore, measurement of the quantity of IL-lu se creted in the culture medium is just one of a host of methods for the evaluation of the anti-inflammatory effects of levan. After inducing primary skin irritation with 0.05% SLS in 3-D artificial skin, 0.01 mg/ml and 0.05 mg/ml of levan solution were applied, and we subsequently measured the quantity of interleukin-la. secreted. Artificial skin treated with 0.01 mg/ml and 0.05 mg/ml of levan exhibited decrements in the quantity of IL-lu, as opposed to the artificial skin, which had not been treated with levan (Figure 8). Taking the above into account, our results suggest that levan exerts an emollient effect during skin irritation caused by irritants. However, the exact mechanism underlying this phenomenon is currently unclear. Despite the high molecular weight of levan, it still exhibits cell proliferation and anti-inflammatory effects. This may be attributable to its penetrative ability, which is in itself due to a much smaller particle size distribution (ranging from about 170 to 300 nm) than is associated with other polysaccharides. CONCLUSIONS In this study, we have determined some of the relevant cosmeceutical properties of levan, including its moisturizing effect, cell cyrotoxicity, cell proliferation effect, and anti inflammation effect. Levan manifested a moisturizing effect highly reminiscent of that
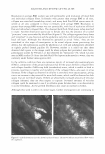
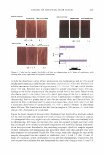
COSMECEUTICAL PROPERTIES OF LEVAN 80 �----------------------, - 70 8 60 QJ r/) ca � 50 tS I �l [ I J (a) r--1 I r - I I 1 (b) (c) (d) Concentration (w/v%) 405 Figure 8. Anti-inflammation effect of levan in 3-D artificial skin: (a) control (b) 0.05% SDS (c) 0.05% SDS + 0.01 mg/ml of levan (d) 0.05% SDS + 0.05 mg/ml of levan. The data are expressed as mean values (± standard deviations) of four experiments. of hyaluronic acid, and also exhibited effects on the proliferation of human fibroblast and keratinocytes reminiscent of hyaluronic acid. Moreover, in our cell proliferation test on 3-D bio-artificial skin, after the induction of primary skin inflammation using 0.05% SLS, cell proliferation on the 3-D artificial skin in the presence of levan was determined to be much more pronounced than in the absence of levan (SLS treatment only). In our anti-inflammation test, the quantity of IL-la. secreted in the 3-D artificial skin treated with levan was shown to be less than that of the 3-D artificial skin treated only with SLS. As a result of these studies, levan was found to exert an anti-inflammatory effect against inflammatory reactions to skin irritants. Levan was also shown to exert a cell prolifera tion effect in bio-artificial skin. We also evaluated levan with regard to its safety and lack of toxicity in a series of safety tests that involved fibroblasts. Our results indicate that levan might, indeed, prove to be a useful and safe cosmeceutical agent. REFERENCES (1) R. Dedonder, Levansucrase from Bacillus subtiliJ, Methods Enzymol., 8, 500-505 (1966). (2) G. D. Bonnett, I. M. Sims, R. J. Simpson, and A. J. Cairns, Structural diversity of fructan in relation to the taxonomy of the Poaceae, New Phytologist, 136, 11-17 (1997). (3) N. C. Carpita, J. Kanabus, and T. L. Housley, Linkage structure of fructan and fructan oligomers from Triticum aestivum and Festuca arundinacea leaves,]. Plant Physiol., 134, 162-168 (1989). (4) G. A. F. Hendry and R. K. Wallace, "The Origin, Distribution, and Evolutionary Significance of Fructan," in Science and Technology of Fructans, M. Suzuki and N. J. Chatterton, Eds. (CRC Press, Boca Raton, FL, 1993), pp. 119-139. (5) E. A.H. Pilon-Smits, M. J.M. Ebskamp, M. J. Paul, M. J. W. Jeucen, P. J. Weisbeek, and S. C. M. Smeekens, Improved performance of transgenic fructan-accumulating tobacco under drought stress, Plant Physiol., 107, 125-130 (1995). (6) M. J. Pabst, Levan and levansucrase of Actinomyces viscosus, Infect. Immun,, 15, 518-526 (1977). (7) T. N. Warner and C.H.Miller, Cell-associated levan of Actinomyces viscosus, Infect. Immun., 19, 711-719 (1978). (8) K. Tanaka, T. Karigane, S. Fujii, T. Chinzaka, and S. Niagamura, Intermolecular fruccosyl and
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)