FADING OF ARTIFICIAL HAIR COLOR 413 treatment solutions, the hair displayed a noticeable deposit of the photofilter on the surface. Benzophenone-4-ZnO combinations. (a) Control: 0.5 g PEG-20 stearate, 0.75 g glyceryl stearate (and) laureth-23 were added to 20 g of deionized water. The dispersion was heated to 50°-60°C with stirring until the emulsifiers melted or dissolved. The solution was cooled to room tem perature, 0.25 g of preservative (propylene glycol and diazolidinyl urea and propyl paraben) was added, and the formulation mass was brought to 25 g with deionized water. (b) 2% ZnO: 0.5 g PEG-20 stearate, 0.75 g glyceryl stearate (and) laureth-23, and 0.50 g ZnO were added to 20 g of deionized water. The dispersion was heated to 50°-60°C with stirring until the emulsifiers melted or dissolved. The solution was cooled to room temperature, 0.25 g of preservative (propylene glycol and diazoli dinyl urea and propylparaben) was added, and the formulation was brought to 2 5 g with deionized water. The formulation needed to be shaken before use due to the settling of the ZnO. (c) 2% Benzophenone-4: 0.5 g PEG-20 stearate, 0.75 g glyceryl stearate (and) laureth- 23, 0.50 g benzopheneon-4, and 1.60 g lM NaOH were added to 20 g of deionized water. The dispersion was heated to 50°-60°C with stirring for 15 min, and then the solution was cooled to room temperature, 0.25 g of preservative (propylene glycol and diazolidinyl urea and propylparaben) was added, and the formulation was brought to 25 g with deionized water. (d) 1 % Benzopheneone-4-1 % ZnO: 0.5 g PEG-20 stearate, 0.75 g glyceryl stearate (and) laureth-23, 0.25 g benzophenone-4, 0.8 g lM NaOH, and 0.25 g ZnO were added to 20 g of deionized water (pH was about 7). The dispersion was heated to 50°-60°C with stirring until the emulsifiers melted or dissolved. The solution was cooled to room temperature, 0.25 g of preservative (propylene glycol and diazoli dinyl urea and propylparaben) was added, and the formulation was brought to 25 g with deionized water. The formulation needed to be shaken before use due to the settling of the ZnO. (e) Benzophenone-3: 1-5% w/w EtOH solutions were employed with 2-g hair tresses treated with 1 g of solution. (f) Benzophenone-3-ZnO: 0.75 g isocetyl alcohol, 0.50 g cetearyl alcohol and ce teareth-20, 0.75 g benzophenone-3 (Escalol 560), and 0.75 g ZnO were stirred in 20.50 g EtOH. After dissolution of the surfactants, 2.50 g of cyclomethicone was added. Products based on benzophenone-3 (Escalol 560, ISP), ZnO, and control systems were prepared according to the same recipe. (g) Benzophenone-4-octyl methoxy cinnamate (OMC)-ZnO: 3% UV absorber (OMC, benzophenone-4, ZnO, or 1.5% OMC or 1.5% benzophenone-4 with 1.5% ZnO), 1 % butylated PVP (Ganex P-904 LC, ISP), 2% cetearyl alcohol (Emulgade 1000 NI, Cognis), and 0.5% preservative (Liquid Germall Plus, ISP). The preservative was a mixture of propylene glycol, diazolidinyl urea, and iodopropynyl butylcarba mate. OMC (Escalol 5 5 7) and benzophenone-4 (Escalol 5 77) were ISP products. Micronized ZnO was from Presperse. (h) Dimethylpabaimidopropyl laurdimonium tosylate-benzophenone-3: dimethylpab amidopropyl laurdimonium tosylate (Escalol HP-610), 1 % isocetyl alcohol (Cera phyl ICA, ISP), 0.4% benzophenone-3, 1 % propylene glycol, 0.4% dimethiconol
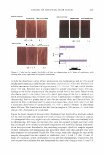
414 JOURNAL OF COSMETIC SCIENCE and cyclopentasiloxane (Si-Tee™ GF 3092), 10% phenyl trimethicone (Si-Tee™ PTM 20), 3% amodimethicone (Si-Tee™ AM 652), 0.1 % cyclopentasiloxane (Si Tec™ CM 040), 29% tocopheryl acetate, 0.1 % SD-40 Alcohol, 55%. RESULTS AND DISCUSSION COLOR FADING BY SHAMPOOING VS IRRADIATION AND SHAMPOOING It is commonly observed that shampooing and solar exposure of dyed hair leads to a noticeable color loss, even for hair treated with permanent hair dyes. This effect is dependent upon the hair color shade and, consequently, the composition of the dyeing system employed in a hair-color formulation (12). The most pronounced fading effects are typically observed for red shades such as light auburn, medium auburn, and dark auburn. Traditional compositions of such hair dyes are based on 4-amino-2-hydroxy toluene, 1-naphtol, or 2-methyl-1-naphthol as red shade couplers (12,13). More vivid red colors can be obtained by using pyrazole derivatives such as 1-hydroxyethyl-4,5- diamino pyrazole sulfate, which act as primary intermediates (12). Other novel inter mediates, such as phenyl methyl pyrazolone and 6-hydroxyindole, can serve as couplers (14,15). In order to compare the color changes from shampooing and exposure to sunlight, we analyzed the samples of three types of hair, which included natural white, dark brown, and bleached hair, all dyed with a dark auburn shade of a commercially available hair color based on non-pyrazole technology (I). One set of samples was shampooed ten times without exposure to sunlight, in which case the hair samples were allowed to dry between each shampooing. Another set of samples was exposed to 32 hours of irradiation and shampooed after every eight hours of exposure. Figure 4 shows the total color change in terms of dB values for hair after the completion of treatments. It is evident that the color variation depends on the type of hair. Washing alone produced the same extent of color change, with dB values of 1.7-1.8 in bleached and white hair. There was less color variation in shampooed dark brown hair subjected to shampooing alone. In contrast to this, dyed white hair underwent very significant color fading (dB = 8.1) as a result of light exposure and shampooing. Most likely, this can be ascribed to the limited pho tostability of the hair-color chromophores and the lack of the photoprotective effect of a natural pigment such as melanin. Additionally, white hair (Piedmont hair) is lightened as a result of irradiation, which might be a contributing factor to the observed large photofading effect. Photo-irradiation/shampooing of bleached hair resulted in a smaller color loss. It is possible that such a result is the consequence of two reactions occurring in bleached hair. First is the fading of the artificial hair color and second is the darkening of background hair as a result of the recondensation of residual melanin products that remain in hair after bleaching. Such a phenomenon was observed and reported for photo-irradiated bleached hair (16). In contrast to this, highly pigmented brown hair showed only small color variation, with a dB value of about 1, after 32 hours of irradiation and four shampooings. Further details of color changes are given in Figures 5a and 56, which show the absor bance spectra of natural white and bleached hair before and after dyeing. The figures also
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)