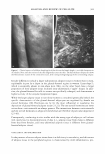
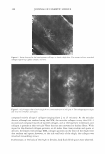
a) Initial FADING OF ARTlflCIAL HAIR COlOR :t6 Ho, s 32 Konn b) I •. ,.,..,, Tol.l,I Cr.lor Clil .. ..,. ■lrnnm.mcrrt, IC,IV111,t111h:a,-w.1ITil:ti 10 12 Fi ure 6. (a) Loss of hair color for •chite hair dyed with a medium auburn dy. and exposed to irradia1ion for 16 and 32 hours. The top-row sample was only exposed to irradiation, while the bottom-row sample was shampooed after every eight hours of irradiation. (b) Color change parameter (dE) for hair shown in (a). The fi�ure also shows the dE values for shampoo cl-only hair (samples placed in the weatherometer, ,1,,·hich , ·as covered and not exposed to radiation) and hair shampool.'d after every eight hours. after 16 and 32 hours, while for areas of the samples chat ·were irradiated and shampooed th mtal color changes wer 7. 3, and 10. 7 aft ,r 16 and 32 hours of irradiation. In an addicional experimen -, mployin .,. another batch of Piedmont hai dyed ,.,,1ith a medium auburn shade of hair color, JE and dC for shampooed but not irradiated areas were 3.6 ± , ).7 and L ± L4, respectivdy, after 32 hours of weatherin • (four shampooing.). :for irradiated and shampooed areas, the changes were 10. ± .7 and 2.7 ± ( .4 for dE and dC, respectively. Thus, the combination of irradiation and shampooing leads to the greate ·c .loss of rnlor (dE), with some change in color shade (c/C). hile shampooing alone produces a relatively small color loss, additional irradiation greatly enhances chis effoct. Sud1 a resul may noc b · surpri .in .,. , since c 'I osure t irradiation i known co damage the I 'ir fiber and increase its po ·osity, makin , the lo· s of larger dye moJecufo by shampooing easier ( 1 7, 18). Finally, coJor fading experiments were performed on white hair treated ,vith pyrazole and pyrnzolnne-concaining intense red and dark auburn shades of hair color (l V and V). lntense red {JV) and dark auburn (V) formulations produce hair color characterized by tristimulus parameters L = 31.2 ± 0.6/a = 30.0 ± 0.2/b = 9.5 ± 0. l and l = 2- .0 ± 1. /a = 7. 7 ± 0.03/b = 7 .0 ± 0.4, re:specrively. After ten shampooings, dB was 4. 7 ± 1.3 and 5.6 ± LO for (IV) and (V) treated hair, respectivdy. Both values are si ·•nificantly higher than those of the corresponding color changes measured for non-pyrazole dyed nacuraJ white hair (l◄igure 4). Similarly, after 32 hours of irradiation/shampooing dE was l .0 ± 2.4 and 16.5 ± 1.3 for (IV) ,nd V), respectively, which shows greater color loss for pyrazole-based permanent hair dyes. In addition to tl1is, the fading of hair coloration can be measured in terms of dC, which m asures a change in color shade. l'hus, after ten shampooings, dC was 1.4 ± 0.5 and 2.1 ± 0.7 for hair treated witl (IV) and (V), respectively. As a result of irradiation, the change in color shade becomes much larger, especially for product OV). The observed dC values were 10.7 ± 0.6 and 3.8 ± 0.5, for (IV) and (V), respectively, after 32 hours of irradiation/shampooing.
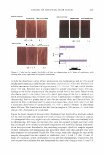
418 JOURNAL OF COSMETIC SCIENCE The observed difference in shampoo fading of hair treated with product (IV), containing 1-hydroxyethyl-4,5-diamino pyrazole sulfate, and hair treated with products (I) or (II) can be probably explained by the higher water solubility of the dye products formed in hair as a result of the use of (IV). One should also point out that the mechanism of reactions involving pyrazolone coupler and its role in the formation of red color was not fully elucidated (12). It was suggested that the material does not participate in the final color formation. An additional complication in the interpretation of the data is the use of ethanolamine and ammonia as alkalizing agents in formula (V), which may affect the dye penetration into the hair. COLOR FADING BY UVB AND UV A It is a common practice to include UV absorbers in hair-care formulations designed for treatment of dyed hair. While visible light can also cause fading, visible-light absorbers are colored, which precludes their use in commercial products. The relative color-fading efficacy of various segments of solar radiation (UVA [320-400 nm}, UVB [280-350 nm}, visible [370-780 nm}, and IR [750-2800 nm}) was investigated by Hoting and Zimmerman for red-colored hair (6). They found that the photo-fading efficiency per 1 W/m2 of light intensity (calculated as dB/(light intensity [W/m2})) is the greatest for UVB (0.38 m2/W) and the smallest for IR (0.0019 m2/W), with a relative efficiency varying in the following order: UVB UVA Vis IR. However, considering the relative intensity of various portions of radiation, which were chosen to approximate the intensities of natural summer sunlight in central Europe, the actual photo-fading effect expressed as dB equaled 8.5, 3.8, 0.95, and 0.84 for hair subjected to visible, UVA, UVB, and IR light irradiation, respectively. Thus the contribution of UVB and UVA to the total photo-fading effect is about 34%. In order to determine if filtering off UV radiation from natural light would result in noticeable color protection, we irradiated hair with simulated solar light passing through special filters such as a Schott glass-type GG 420 UV filter, which effectively blocks UV light (-0% transmission below 390 nm), and the quartz filter, which is nearly transparent to light with a wavelength above 290 nm. White hair treated with a commercial medium auburn dye (II) was employed in these experiments. Figure 7 shows the total color change of hair, dB1 after 16, 32, and 48 hours of irradiation. Hair samples covered with the UV filter showed -30-60% less color loss and were noticeably darker and redder than the samples covered with the quartz plates. The amount of protection offered by the UV filter is calculated from the formula 100 x (dBc - dBp) %Protection = d Ee (3) where dB c and dB p are the total color changes after the irradiation of unprotected (quartz filter) and UV filter-protected hair, respectively. Results presented in Figure 7 refer to hair samples that were irradiated without shampooing. It should be noted that the amount of protection varied in the range of 27% to 63%, depending upon the time of exposure. Longer irradiation times led to smaller photoprotection values. This result could be expected as a consequence of the existence of a limiting value of residual concentration of chromophores in a hair color, which are gradually depleted during photo-exposure (at sufficiently long exposures the coloration should completely fade
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)