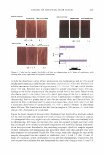
a) 12 7 0 10 FADING OF ART FICI .... HAIR COLOR 419 20 JO 40 50 lrr,adlation 1ime (Hr:&) 60 b) frer 8 hrs irradiafon Irradia1ed through quartz Irradiated through filter blocking UV light "igun: 7. Total color loss for white hair, dyed with medium auburn dye (a) and irradiated through a quartz filter and a filter completely absorbing UV light (b). away, and% protection would be reduced m zero). In summary, the present data confirm the previous suggestion that blocking UV light can ·ignificantly limit artifici, I hair color fading. THEORETI 'AL AND EXPERIMENTAL ANAI.YSIS OF PHOH R TECTION B UVA AND fJVB PHOT HlTER, The tht:oretical extent of phoroprotection by a formulation, containing phow-absorbers 'nd evenly disnjbuted on h, ir, can b� assessed by calcula1ting the percentage of UV lighr it absorbs. It can be shown that the attemntinn of li :i-ht, at a given wavelength, b, a multi-component photofilter , ystem is given by 1:he foJlowing: relationship, which is a modification of Beer's equation: wh. re / /"Ii.) is the ab, orbanc of _pho ofil t r i at wavelength A, l s(A) i the intensity of solar radiation, ep1.) is the molar extinction coefficient of )homfilter i at wavelen , h (A.), m i is the amoun of formulation deposited on hair (gig hair), C is i:he concentm -ion of a photofilter in a formulation (% w/w), J\ is the specific surface area of hair assumed to be 800 (cm2/g), p is the densiry of a dry formulation (assumed to be approximately 1 g/cm3 ), and /W u •i is th molecular weight of an i-�h photofil r. Equation 4 can be used to calculate the fraction of light absorbed at each wavel.ength by a given quantity of absorber on the hair. The total percentage of absorbed UV radiation can be determined by integrating (numerically) equation 4 over th wavelength range 29-0-400 nm. For calculations, values for the solar irradiation on the surface of the earth between ?90 mm and 400 nm were taken from the literature (19). figure 8 shows rhe _percentage of attenuated UVB and UV A light (290�/400 nm) as a function of the
420 JOURNAL OF COSMETIC SCIENCE 9()-.-------------------------------------, 80 -----------------------------1 Avobenzone -a 70+---------------=�-==----------------I CD .cl 0 60+--------------::-.,,,,::.-----------------------1 u, .cl !50-t-------------------------------------1 .c en ���--��-----------�--������-----I ::I Benz-4 :30+--��-----�----:::--o,::::_--------:�---=---------=-:-:-=----------1 ::, · · · · · · · · · -;;e 20+-------.,-----------:::aJll"'l-,::__--:::a.,..=----=--..-----,,------!!..•__.!•:........:.._•_•_._._.---=--:-:-----,=-:--::--:--I CmalTIQOpmpyl Trineth'JI 10 0 0 10 mg absorber/gm hair Amin.Cl 20 30 Figure 8. Calculated proponion of attenuated UVB and UV A light (290--400 nm) as a function of the amount of photo-absorber deposited on the surface of hair. amount of UV photo-absorbers deposited on the hair. The data show that UVB absorbers such as dimethylpabaimidopropyl laurdimonium tosylate, cinnamidopropyl ammonium chloride, and octyl methoxy cinnamate absorb less than 25% of the total UV radiation. This is because UVB (290-320 nm) constitutes only about 9% of the total solar radiation in the UV range. As might be expected, the compounds with a greater UV A absorbance (benzophenone-3, benzophenone-4, and butyl methoxydibenzoylmethane) absorb a greater percentage of the radiation between 290 mm and 400 nm. It has to be stressed that the theoretical assessment of the performance of various photofilters pre sented in Figure 8 assumes that they are characterized by perfect photostability. Such an assumption is justified in the case of materials such as benzophenone-3 and benzophe none-4. For butyl methoxydibenzoylmethane, which is very unstable under irradiation, the results of the calculation probably overestimate the capacity of this compound to absorb UV light under practical usage conditions. In a series of experiments, tresses of natural white hair dyed with a medium auburn dye were treated with leave-in aqueous or ethanol solutions of benzophenone-3 and benzo phenone-4, respectively. Figure 9 presents a gradual decrease in L1E as a function of the concentration of benzophenone-3 after 16 hours of irradiation and two shampooings. Figure 10 is a plot of percent color protection (calculated according to equation 3 from the experimental data) for both benzophenone-3 and benzophnone-4 as a function of the calculated extent of UV attenuation (calculated from spectroscopic data by using equa tion 4) by a given treatment. Extrapolation of the linear least-square fit to the data points suggests 72% color protection for 100% UV attenuation. This is in reasonable agree ment with the 63% protection observed in an experiment in which glass filters were employed to completely eliminate UV radiation (Figure 7), but it has to be kept in mind
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)