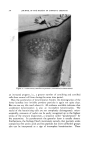
594 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS $G $M Figure 11.--Diagrammatic representation of epidermal ultrastructure (see text for explana- tion). SM: stratum malpighii SL: stratum lucidurn SG: stratum granulosum SC: stratum comeurn substructure has been observed in this matrix in the human footpad and there are only occasional vacuoles which might have been occupied by such components as lipid droplets. The amount of extraneous cellular material in the keratinized cells appears to vary with tissue and species as would be expected from the variation in keratin and cornification be- tween various types of skins. In view of the many observations on the presence of cell membranes in
HUMAN EPIDERMIS REVEALED BY EI.ECTRON MICROSCOPE 595 the S. corneum and Matoltsy's (36) description of "extremely resistant cell membranes" in macerated S. comeurn, it comes as no surprise that these membranes are clearly visualized in electron micrographs. See Fig. 10. The membranes must possess considerable intrinsic electron density to show up with sufficient contrast against the solid matrix of the cells. They are clearly continuous with desmosomes which differ from the des- mosomes of the lower layers in that the dense band of intercellular ma- terial has disappeared and the cell membranes are drawn closer together, being separated now by only about 200 •. Cell membranes are obviously less tightly bound in the areas between desmosomes since they readily separate from one another giving rise to the "prickled" appearance on many occasions. DISCUSSION Judging from the available literature, the two aspects of epidermal differentiation of greatest interest to the cosmetic chemist and the der- matologist are keratinization and desquamation--i.e., it is their ambition to correct aberrations in one or both of these processes. Thus this final discussion will be limited to tonofilaments and desmosomes, those cell components clearly implicated most directly in keratJnization and desqua- marion, respectively. These observations have shown that all cellular components, except tonofilaments, cell membranes and desmosomes, disappear or degenerate before the cells reach the fully keratinized layer. Not much need be said about the tonofilaments to this audience since it is clear that, as the sub- microscopic units of the tonofibrils, they are the site of the "alpha" type fibrous protein, identified by x-ray diffraction, in the Malpighian cell layer. The only tissue from which this protein has been extracted and characterized is the cow's nose (19) but, it is clear (21) that some fibrous protein is present in, and can be extracted from, human material and we may eagerly await the results of this study. In this connection it might be suggested that, in view of many similarities between muscle and epi- dermal cells, it might be worth while to extract tonofilaments with the same methods used for muscle filaments: i.e. maceration of fresh tissue followed by differential centrifugation. Our knowledge of intracellular fibrous proteins of other types is quite extensive and indicates that they may occur fully oriented in living cells. The filaments observed in the electron microscope represent an ordered arrangement of polypeptide chains ("alpha" helices) packed parallel to each other. The constant diameter of the filaments indicates that this is not a haphazard packing but that the filament is a reproducible unit of structure: i.e., the protein must be synthesized in this form in the living cell. Tonofibrils then are bundles of filaments that are grouped together
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)