427 SUSTAINABLE HAIR
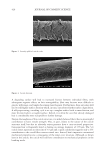
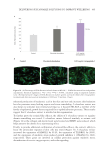
Figure 5 is a frequently used schematic for illustrating the multifaceted structure of hair.8
It shows how α-helical keratin protein chains wrap together to form a coiled rope-like
structure, which further entwines to produce intermediate filaments (frequently described
as microfibrils). These pack within an amorphous keratin matrix (sometimes termed
keratin-associated protein) to form a microfibril (often termed a cortical cell). These all
pack together within the lipid structure of the cell membrane complex (CMC), and the
whole assembly is encased within the outer cuticle. In short, the result is a bio-composite
fiber made primarily out of keratin proteins.
A protein falls into the keratin family due to the presence of the amino acid cystine
somewhere within its structure. The disulfide bond at the heart of this molecule is
thought to be the main crosslinking structure within the protein chains and is believed
to provide most of hair’s permanent structure. Yet this is a relatively reactive moiety. In
permanent waving, this bond is attacked by reducing agents to deconstruct a portion of
the protein structure.9 Attempts are later made to reform this bonding, with the hair in a
new conformation, using oxidizing treatments. Inevitably not all the broken bonds can be
re-formed, and a portion are lost to cysteic acid formation. These bonds can also be directly
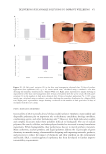
oxidized into cysteic acid via oxidizing treatments.10 For example, bleaching treatments
utilize these chemicals to degrade the melanin pigment granules that provide hair’s natural
color. Similarly, these bonds can be photochemically oxidized under the action of the sun’s
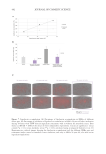
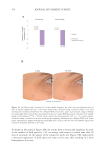
UV rays.11 The obvious implication of losing strength-supporting bonds is that the hair is
less well-structured, and its strength is compromised (examples will be presented later in
this article). From a practical stance, this might be anticipated to make hair more prone to
breakage.
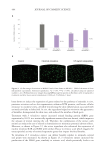
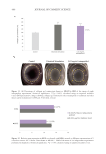
Another consequence of this diminished structuring is that fibers will swell more when
wetted by water.12 It has been suggested that water should be considered part of hair’s
structure, due to the sizable effect its presence has on many of the hair’s properties.13 Simply
Figure 5. Schematic of hair structure. Reproduced from reference 8. Copyright 1991, Elsevier.
Figure 5 is a frequently used schematic for illustrating the multifaceted structure of hair.8
It shows how α-helical keratin protein chains wrap together to form a coiled rope-like
structure, which further entwines to produce intermediate filaments (frequently described
as microfibrils). These pack within an amorphous keratin matrix (sometimes termed
keratin-associated protein) to form a microfibril (often termed a cortical cell). These all
pack together within the lipid structure of the cell membrane complex (CMC), and the
whole assembly is encased within the outer cuticle. In short, the result is a bio-composite
fiber made primarily out of keratin proteins.
A protein falls into the keratin family due to the presence of the amino acid cystine
somewhere within its structure. The disulfide bond at the heart of this molecule is
thought to be the main crosslinking structure within the protein chains and is believed
to provide most of hair’s permanent structure. Yet this is a relatively reactive moiety. In
permanent waving, this bond is attacked by reducing agents to deconstruct a portion of
the protein structure.9 Attempts are later made to reform this bonding, with the hair in a
new conformation, using oxidizing treatments. Inevitably not all the broken bonds can be
re-formed, and a portion are lost to cysteic acid formation. These bonds can also be directly
oxidized into cysteic acid via oxidizing treatments.10 For example, bleaching treatments
utilize these chemicals to degrade the melanin pigment granules that provide hair’s natural
color. Similarly, these bonds can be photochemically oxidized under the action of the sun’s
UV rays.11 The obvious implication of losing strength-supporting bonds is that the hair is
less well-structured, and its strength is compromised (examples will be presented later in
this article). From a practical stance, this might be anticipated to make hair more prone to
breakage.
Another consequence of this diminished structuring is that fibers will swell more when
wetted by water.12 It has been suggested that water should be considered part of hair’s
structure, due to the sizable effect its presence has on many of the hair’s properties.13 Simply
Figure 5. Schematic of hair structure. Reproduced from reference 8. Copyright 1991, Elsevier.