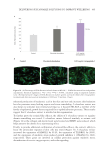
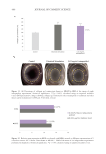
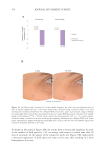
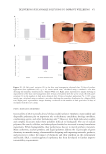
400 JOURNAL OF COSMETIC SCIENCE
for the skin over neutral and alkaline pH cleansers. This aspect is reviewed in detail and
the key findings are summarized by Hawkins et al.124
Common soap bars with alkyl carboxylate-based surfactants are formulated at alkaline pH
conditions as high as 10–11.This is mainly because of the insolubility of soap molecules in
bar formulations below pH 9.0. By using organic counterions, such as tri-ethanol amine,
it is possible to lower the pH of soaps to about 8.4. With the introduction of neutral pH
syndet (synthetic detergent) bars in the mid-50s with acyl isethionate as the main surfactant,
neutral pH cleaning became popular. Importantly, Frosch and Kligman compared the
irritation potential of several alkaline soap bars to the isethionate based syndet bar in their
classic study using their soap chamber test, and showed that the neutral pH syndet bar was
significantly milder than alkaline soap bars.25 This has been confirmed with several less
aggressive cleansing protocols,26 including the popular FCAT methodology for assessing
the mildness of cleansing products.27
With the recent advances in our understanding of the role of pH in maintaining a healthy
SC barrier and the benefits of hyper-acidified leave-on products on skin barrier recovery
after tape striping, there has been an increase in formulating cleansing formulations at
skin pH.124 Contrary to expectations, Hawkins et al. showed that marketed syndet bars
with synthetic detergents such as alkyl sulfosuccinate as the main surfactant and prototype
bars formulated with acyl isethionate as the main surfactant under skin pH conditions
were harsher than the neutral pH acyl isethionate bars.124 Similarly, prototype liquid
formulations with sodium laureth sulfate as the main anionic surfactant formulated at skin
pH and neutral pH conditions also showed that the acidic pH cleansers were harsher than
their neutral pH counterparts. These results clearly show that simply formulating a product
at skin pH does not ensure enhanced mildness. The authors explained these differences due
to increased binding of anionic surfactants to the SC as the pH is lowered from neutral pH
to acidic pH conditions. The isoelectric point of SC is around pH 4.5. Lowering the pH of
the cleanser from neutral to skin pH conditions will increase the positively charged sites on
keratin, where anionic surfactants can bind strongly, leading to increased damage and skin
irritation. These results clearly show that it is important to understand the interactions
of surfactants and all other ingredients in a formulation with the SC under different pH
conditions to determine the relative benefits of skin pH versus other conditions for ensuring
mildness of a product. It is also important to recognize that these results do not imply that
it is not possible to formulate a skin cleansing product under skin pH conditions, but that
it requires surfactants that are mild to skin under low pH conditions.
The interest in formulating skincare and skin cleansing products under skin pH conditions
will continue in the coming years. It is therefore important for formulators to understand
the effect of pH on the interaction of their ingredients and the overall formulation with the
SC under skin pH conditions rather than assuming that any product formulated under skin
pH conditions will be mild to the SC.
CONCLUSION
Cosmetic and personal care formulations contain a wide range of ingredients including
surfactants, emulsifiers, penetration enhancers, emollients, occlusives, physiological and
nonphysiological lipids, and bio-actives ingredients. The interactions of these ingredients
with the SC will determine their ability to deliver the intended benefits without
for the skin over neutral and alkaline pH cleansers. This aspect is reviewed in detail and
the key findings are summarized by Hawkins et al.124
Common soap bars with alkyl carboxylate-based surfactants are formulated at alkaline pH
conditions as high as 10–11.This is mainly because of the insolubility of soap molecules in
bar formulations below pH 9.0. By using organic counterions, such as tri-ethanol amine,
it is possible to lower the pH of soaps to about 8.4. With the introduction of neutral pH
syndet (synthetic detergent) bars in the mid-50s with acyl isethionate as the main surfactant,
neutral pH cleaning became popular. Importantly, Frosch and Kligman compared the
irritation potential of several alkaline soap bars to the isethionate based syndet bar in their
classic study using their soap chamber test, and showed that the neutral pH syndet bar was
significantly milder than alkaline soap bars.25 This has been confirmed with several less
aggressive cleansing protocols,26 including the popular FCAT methodology for assessing
the mildness of cleansing products.27
With the recent advances in our understanding of the role of pH in maintaining a healthy
SC barrier and the benefits of hyper-acidified leave-on products on skin barrier recovery
after tape striping, there has been an increase in formulating cleansing formulations at
skin pH.124 Contrary to expectations, Hawkins et al. showed that marketed syndet bars
with synthetic detergents such as alkyl sulfosuccinate as the main surfactant and prototype
bars formulated with acyl isethionate as the main surfactant under skin pH conditions
were harsher than the neutral pH acyl isethionate bars.124 Similarly, prototype liquid
formulations with sodium laureth sulfate as the main anionic surfactant formulated at skin
pH and neutral pH conditions also showed that the acidic pH cleansers were harsher than
their neutral pH counterparts. These results clearly show that simply formulating a product
at skin pH does not ensure enhanced mildness. The authors explained these differences due
to increased binding of anionic surfactants to the SC as the pH is lowered from neutral pH
to acidic pH conditions. The isoelectric point of SC is around pH 4.5. Lowering the pH of
the cleanser from neutral to skin pH conditions will increase the positively charged sites on
keratin, where anionic surfactants can bind strongly, leading to increased damage and skin
irritation. These results clearly show that it is important to understand the interactions
of surfactants and all other ingredients in a formulation with the SC under different pH
conditions to determine the relative benefits of skin pH versus other conditions for ensuring
mildness of a product. It is also important to recognize that these results do not imply that
it is not possible to formulate a skin cleansing product under skin pH conditions, but that
it requires surfactants that are mild to skin under low pH conditions.
The interest in formulating skincare and skin cleansing products under skin pH conditions
will continue in the coming years. It is therefore important for formulators to understand
the effect of pH on the interaction of their ingredients and the overall formulation with the
SC under skin pH conditions rather than assuming that any product formulated under skin
pH conditions will be mild to the SC.
CONCLUSION
Cosmetic and personal care formulations contain a wide range of ingredients including
surfactants, emulsifiers, penetration enhancers, emollients, occlusives, physiological and
nonphysiological lipids, and bio-actives ingredients. The interactions of these ingredients
with the SC will determine their ability to deliver the intended benefits without