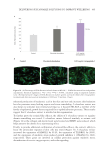
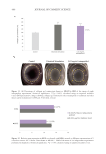
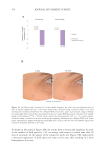
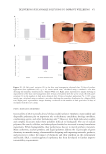
504 JOURNAL OF COSMETIC SCIENCE
in controlling S. aureus growth on skin.76 Barnard et al.77 found that there was a greater
relative abundance of C. acnes phages in healthy skin compared to lesional skin of acne
test subjects. Natarelli and coworkers noted that phage-based therapeutic strategies appear
promising for the treatment of a variety of dermatological conditions.75
MICROBIAL INTERACTIONS WITH THE SKIN
Many researchers have considered the dominant skin bacteria to be commensals.66,68,78
However, information gained over the past several years indicates that some members of
the skin microflora benefit from the nutrients and environment provided by the skin and
the skin benefits by having improved barrier function, an activated SIS, and protection
from colonization by transients and pathogens.79,80 Although “commensalism” describes
the biological interaction in which members of one species gain benefits while other
species neither benefit nor are harmed and “mutualism” describes the biological interaction
between two or more organisms in which each organism has a net benefit, this review
considers commensals to be microorganisms that are either neutral or beneficial to skin.
Orth observed that skin microflora may confer some of the same benefits to skin as
probiotics do in the GI tract.78,81 These benefits are achieved by “colonization resistance”
which helps maintain homeostasis of the skin/skin microbiome ecosystem. Colonization
resistance involves:
1. Growth of commensal microorganisms on skin and mucous membranes lowers the pH,
which makes it more difficult for competing microorganisms (transients and pathogens)
to grow.
2. Commensal skin microflora compete with transients and pathogens for nutrients, which
makes it more difficult for them to grow.
3. Commensal skin microflora compete with transients and pathogens for epidermal
receptor sites and may block their colonization on skin.
4. Association of commensal skin microflora with immunologically competent cells in the
skin, such as keratinocytes and Langerhans cells, may stimulate the SIS so that it is a state
of readiness when stimulated by transients and potentially harmful microorganisms.
5. Crosstalk between the commensal skin microflora and keratinocytes enhances
antimicrobial peptide (AMP) expression by keratinocytes including human β-defensin
2 (HBD2), cathelicidin LL-37, ribonuclease-7, psoriasin (S100A7), and dermicidin.80-83
Table VII
Top 10 Abundant Fungi on Various Skin Sites
Dry Moist Sebaceous Foot
Malassezia restricta Malassezia globosa Malassezia restricta Malassezia restricta
Malassezia globosa Malassezia restricta Malassezia globosa Tricophyton rubrum
Aspergillus tubingensis Tielleti walkeri Malassezia sympodialis Malassezia globosa
Candida parapsilosis Malassezia sympodialis Aureoumbra lagunensis Pyramimonas parkeae
Zymoseptoria tritici Pyramimonas parkeae Tielleti walkeri Trychophyton mentogrophytes
Malassezia sympodialis Parachlorella kessleri Pycnococcus provosolii Parachlorella kessleri
Epidermophyton floccosum Aspergillus tubingensis Gracilaria tenuistipitata Aspergillus tubingensis
Pyramimonas parkeae Zymoseptoria tritici Pyramimonas parkeae Zymoseptoria tritici
Nannizzia nana Nephroselmis olivacea Parachlorella kessleri Gracilaria tenuistipitata
Parachlorella kessleri Cyanophora paradoxa Leucocytozoon majoris Nephroselmis olivacea
*Table adapted from Byrd, Belkaid, and Segre.74
in controlling S. aureus growth on skin.76 Barnard et al.77 found that there was a greater
relative abundance of C. acnes phages in healthy skin compared to lesional skin of acne
test subjects. Natarelli and coworkers noted that phage-based therapeutic strategies appear
promising for the treatment of a variety of dermatological conditions.75
MICROBIAL INTERACTIONS WITH THE SKIN
Many researchers have considered the dominant skin bacteria to be commensals.66,68,78
However, information gained over the past several years indicates that some members of
the skin microflora benefit from the nutrients and environment provided by the skin and
the skin benefits by having improved barrier function, an activated SIS, and protection
from colonization by transients and pathogens.79,80 Although “commensalism” describes
the biological interaction in which members of one species gain benefits while other
species neither benefit nor are harmed and “mutualism” describes the biological interaction
between two or more organisms in which each organism has a net benefit, this review
considers commensals to be microorganisms that are either neutral or beneficial to skin.
Orth observed that skin microflora may confer some of the same benefits to skin as
probiotics do in the GI tract.78,81 These benefits are achieved by “colonization resistance”
which helps maintain homeostasis of the skin/skin microbiome ecosystem. Colonization
resistance involves:
1. Growth of commensal microorganisms on skin and mucous membranes lowers the pH,
which makes it more difficult for competing microorganisms (transients and pathogens)
to grow.
2. Commensal skin microflora compete with transients and pathogens for nutrients, which
makes it more difficult for them to grow.
3. Commensal skin microflora compete with transients and pathogens for epidermal
receptor sites and may block their colonization on skin.
4. Association of commensal skin microflora with immunologically competent cells in the
skin, such as keratinocytes and Langerhans cells, may stimulate the SIS so that it is a state
of readiness when stimulated by transients and potentially harmful microorganisms.
5. Crosstalk between the commensal skin microflora and keratinocytes enhances
antimicrobial peptide (AMP) expression by keratinocytes including human β-defensin
2 (HBD2), cathelicidin LL-37, ribonuclease-7, psoriasin (S100A7), and dermicidin.80-83
Table VII
Top 10 Abundant Fungi on Various Skin Sites
Dry Moist Sebaceous Foot
Malassezia restricta Malassezia globosa Malassezia restricta Malassezia restricta
Malassezia globosa Malassezia restricta Malassezia globosa Tricophyton rubrum
Aspergillus tubingensis Tielleti walkeri Malassezia sympodialis Malassezia globosa
Candida parapsilosis Malassezia sympodialis Aureoumbra lagunensis Pyramimonas parkeae
Zymoseptoria tritici Pyramimonas parkeae Tielleti walkeri Trychophyton mentogrophytes
Malassezia sympodialis Parachlorella kessleri Pycnococcus provosolii Parachlorella kessleri
Epidermophyton floccosum Aspergillus tubingensis Gracilaria tenuistipitata Aspergillus tubingensis
Pyramimonas parkeae Zymoseptoria tritici Pyramimonas parkeae Zymoseptoria tritici
Nannizzia nana Nephroselmis olivacea Parachlorella kessleri Gracilaria tenuistipitata
Parachlorella kessleri Cyanophora paradoxa Leucocytozoon majoris Nephroselmis olivacea
*Table adapted from Byrd, Belkaid, and Segre.74