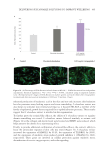
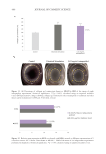
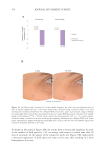
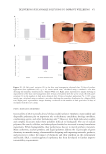
450 JOURNAL OF COSMETIC SCIENCE
medium, glucose, and palmitic acid were used as a metabolic stress control. After 48 hours
of incubation with treatments, a couple of washes were made with PBS (phosphate-buffered
saline, Sigma-Aldrich, St. Louis, MO, USA), and microscopic images and measurements
were performed with a high-content screening system (Operetta® High-Content Imaging
System, PerkinElmer, Inc., Waltham, MA, USA) and processed using Harmony® software
(PerkinElmer, Inc., Waltham, MA, USA). Fluorescence intensities were measured using a
Hoechst33342 channel (Emission: 410-480 nm) and were corrected using the cell number
determined by DPC (Digital Phase Contrast). These values were normalized by the control
or metabolic stress control when applicable. Three independent assays were performed in
four wells per condition. Statistical significance was calculated using an unpaired student’s
t test.
INHIBITION OF IL1ALPHA RELEASE IN HUMAN EPIDERMAL KERATINOCYTES
HEKa cells were seeded and incubated in a culture medium following the same protocol
described in the previous experiment (Inhibition of HMGB1 stressorin release). Cells
treated with the culture medium, glucose, and palmitic acid were used as a metabolic
stress control. Cells treated with the culture medium alone were used as a control. Cells
treated with a specific RAGE-blocking antibody (ABCAM) were used as a control for
IL1alpha release inhibition. After 24 hours of incubation, the supernatants were collected,
and a protease inhibitor cocktail (Merk Life Science) was added they were then kept at
-80°C until the supernatants were analyzed to determine the IL1alpha level by AlphaLISA
(PerkinElmer, Inc., Waltham, MA, USA) according to the manufactureŕs protocol. These
results were normalized with the total viable cell number for each condition calculated
by the PrestoBlueTM staining assay (Thermo Fisher Scientific, Waltham, MA, USA). The
obtained values were normalized versus the control or metabolic stress control as applicable.
Each condition was tested in three to four independent experiments and in biological
triplicates. Statistical significance was calculated using an unpaired student’s t test.
QUANTIFICATION OF MBNL1 PROTEIN LEVELS ON HUMAN SKIN FIBROBLASTS
Electrical stimulation of fibroblasts was performed by seeding cells in 12-well plates over
sterile glass slides and incubating them at 37°C and 5% CO
2 for 48 hours. The glass
slides were transferred to 6-well plates and were electro-stimulated (1.5 V, 70 mV/mm,
1000 µA) for 1 hour using a C-dish device (IonOptix, Westwood, MA, USA) at room
temperature. An identical 6-well plate with nonelectrostimulated cells were incubated
at room temperature for 1 hour (control). After electro-stimulation, glass slides were
transferred again to a 12-well plate and incubated at 37°C and 5% CO
2 for 24 hours. Three
different replicates were done for each condition. Tetrapeptide-1 treatment was performed
by seeding cells in 96-well plates and incubating them at 37°C and 5%CO
2 for 48 hours.
After the incubation time, cells were treated with tetrapeptide-1 at 0.01 mg/mL, 0.1 mg/
mL, and 0.5 mg/mL, respectively, for 24 hours. Cells incubated with the medium alone were
used as a control. Three different replicates were done for each condition. After 24 hours
of incubation, cells were washed twice with PBS, fixed with 4% PFA (paraformaldehyde
solution) for 15 minutes, and washed twice more. After that, cells were permeabilized with
1% X-triton solution for 15 minutes and blocked with a 5% BSA (bovine serum albumin)
solution for 1 hour. Cells were then incubated with a 1/20 dilution of the primary antibody
medium, glucose, and palmitic acid were used as a metabolic stress control. After 48 hours
of incubation with treatments, a couple of washes were made with PBS (phosphate-buffered
saline, Sigma-Aldrich, St. Louis, MO, USA), and microscopic images and measurements
were performed with a high-content screening system (Operetta® High-Content Imaging
System, PerkinElmer, Inc., Waltham, MA, USA) and processed using Harmony® software
(PerkinElmer, Inc., Waltham, MA, USA). Fluorescence intensities were measured using a
Hoechst33342 channel (Emission: 410-480 nm) and were corrected using the cell number
determined by DPC (Digital Phase Contrast). These values were normalized by the control
or metabolic stress control when applicable. Three independent assays were performed in
four wells per condition. Statistical significance was calculated using an unpaired student’s
t test.
INHIBITION OF IL1ALPHA RELEASE IN HUMAN EPIDERMAL KERATINOCYTES
HEKa cells were seeded and incubated in a culture medium following the same protocol
described in the previous experiment (Inhibition of HMGB1 stressorin release). Cells
treated with the culture medium, glucose, and palmitic acid were used as a metabolic
stress control. Cells treated with the culture medium alone were used as a control. Cells
treated with a specific RAGE-blocking antibody (ABCAM) were used as a control for
IL1alpha release inhibition. After 24 hours of incubation, the supernatants were collected,
and a protease inhibitor cocktail (Merk Life Science) was added they were then kept at
-80°C until the supernatants were analyzed to determine the IL1alpha level by AlphaLISA
(PerkinElmer, Inc., Waltham, MA, USA) according to the manufactureŕs protocol. These
results were normalized with the total viable cell number for each condition calculated
by the PrestoBlueTM staining assay (Thermo Fisher Scientific, Waltham, MA, USA). The
obtained values were normalized versus the control or metabolic stress control as applicable.
Each condition was tested in three to four independent experiments and in biological
triplicates. Statistical significance was calculated using an unpaired student’s t test.
QUANTIFICATION OF MBNL1 PROTEIN LEVELS ON HUMAN SKIN FIBROBLASTS
Electrical stimulation of fibroblasts was performed by seeding cells in 12-well plates over
sterile glass slides and incubating them at 37°C and 5% CO
2 for 48 hours. The glass
slides were transferred to 6-well plates and were electro-stimulated (1.5 V, 70 mV/mm,
1000 µA) for 1 hour using a C-dish device (IonOptix, Westwood, MA, USA) at room
temperature. An identical 6-well plate with nonelectrostimulated cells were incubated
at room temperature for 1 hour (control). After electro-stimulation, glass slides were
transferred again to a 12-well plate and incubated at 37°C and 5% CO
2 for 24 hours. Three
different replicates were done for each condition. Tetrapeptide-1 treatment was performed
by seeding cells in 96-well plates and incubating them at 37°C and 5%CO
2 for 48 hours.
After the incubation time, cells were treated with tetrapeptide-1 at 0.01 mg/mL, 0.1 mg/
mL, and 0.5 mg/mL, respectively, for 24 hours. Cells incubated with the medium alone were
used as a control. Three different replicates were done for each condition. After 24 hours
of incubation, cells were washed twice with PBS, fixed with 4% PFA (paraformaldehyde
solution) for 15 minutes, and washed twice more. After that, cells were permeabilized with
1% X-triton solution for 15 minutes and blocked with a 5% BSA (bovine serum albumin)
solution for 1 hour. Cells were then incubated with a 1/20 dilution of the primary antibody