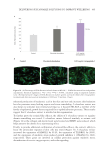
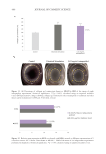
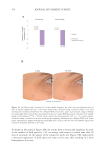
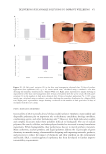
492 JOURNAL OF COSMETIC SCIENCE
The physical and chemical requirements for microbial growth include suitable temperature,
pH, a
w ,substrates/nutrients, oxygen (or the proper oxidation/reduction potential), organic
growth factors (which are needed by some bacteria), and freedom from harmful radiation
and chemicals (e.g., preservatives and antibiotics). Cosmetic ingredients and finished
products provide a wide range of organic and inorganic compounds that will support
microbial growth when water is present. Interestingly, the principles of preservation are
just the opposite of the requirements for microbial growth, which means that application
of these principles prevents bacteria from having access to the things they need, and use of
inhibitory agents (i.e., preservatives/biocides) causes their death.11 Use of these principles to
develop adequately preserved products helps manufacturers meet environmental concerns
and consumer demands for products that are free of chemicals recognized as preservatives.31
Preventing unnecessary microbial load, or bioburden, of the process stream is an
important consideration for making products in accordance with GMPs. This is the reason
manufacturers have microbial specifications (i.e., limits) for raw materials—to minimize
the microbial load in order to maintain microbiological control of the process, because
smaller populations of any specific microorganism require shorter times to kill than
larger populations with any given killing treatment (e.g., heat, chlorination, preservatives,
etc.).11Suppliers of cosmetic ingredients control the microbial load by removal of “bad”
components and avoiding contamination of raw materials obtained from natural sources,
including botanicals and materials obtained in mining operations (e.g., talc, clays, and salts).
The time/temperature parameters involved in preparing raw materials that have aqueous
steps in preparation (e.g., plant extracts and protein powders) are controlled to prevent
microbial growth. Finished product manufacturers prevent microbial growth by use of
adequate preservative systems in products and use of validated manufacturing procedures,
often using Hazard Analysis Critical Control Point (HACCP),32 to ensure microbiological
control of each step in manufacturing from receipt and storage of raw materials, cleaning
and sanitization of equipment, production of the product, packaging, product testing,
and storage prior to shipment to the trade.33 Selection of the proper antimicrobials and
concentrations needed are critical for adequate product preservation, and understanding the
characteristics of the ideal preservative provides the basis for this.
THE IDEAL PRESERVATIVE
There has been an evolution of the characteristics of the “ideal preservative” that have been
identified over the past 75 years to incorporate both positive and negative characteristics,
including inactivating microorganisms quickly enough to prevent adaptation, no cross-
resistance with other preservatives or antibiotics, no negative environmental issues, no
adverse effects on the skin microbiome, and sustainability. The ideal preservative is one that
is both safe and effective for killing all types of microorganisms in all types of formulations,
but it has never been found.14 The reason for this is that no single preservative meets all the
characteristics of the ideal preservative, which are:
• It should have a broad spectrum of activity, which means that it would be effective
against all types of microorganisms so that it could be used as the only preservative in
a formulation.
• It should be effective at low concentrations and be active over the range of pH values
used in cosmetics and drugs.
The physical and chemical requirements for microbial growth include suitable temperature,
pH, a
w ,substrates/nutrients, oxygen (or the proper oxidation/reduction potential), organic
growth factors (which are needed by some bacteria), and freedom from harmful radiation
and chemicals (e.g., preservatives and antibiotics). Cosmetic ingredients and finished
products provide a wide range of organic and inorganic compounds that will support
microbial growth when water is present. Interestingly, the principles of preservation are
just the opposite of the requirements for microbial growth, which means that application
of these principles prevents bacteria from having access to the things they need, and use of
inhibitory agents (i.e., preservatives/biocides) causes their death.11 Use of these principles to
develop adequately preserved products helps manufacturers meet environmental concerns
and consumer demands for products that are free of chemicals recognized as preservatives.31
Preventing unnecessary microbial load, or bioburden, of the process stream is an
important consideration for making products in accordance with GMPs. This is the reason
manufacturers have microbial specifications (i.e., limits) for raw materials—to minimize
the microbial load in order to maintain microbiological control of the process, because
smaller populations of any specific microorganism require shorter times to kill than
larger populations with any given killing treatment (e.g., heat, chlorination, preservatives,
etc.).11Suppliers of cosmetic ingredients control the microbial load by removal of “bad”
components and avoiding contamination of raw materials obtained from natural sources,
including botanicals and materials obtained in mining operations (e.g., talc, clays, and salts).
The time/temperature parameters involved in preparing raw materials that have aqueous
steps in preparation (e.g., plant extracts and protein powders) are controlled to prevent
microbial growth. Finished product manufacturers prevent microbial growth by use of
adequate preservative systems in products and use of validated manufacturing procedures,
often using Hazard Analysis Critical Control Point (HACCP),32 to ensure microbiological
control of each step in manufacturing from receipt and storage of raw materials, cleaning
and sanitization of equipment, production of the product, packaging, product testing,
and storage prior to shipment to the trade.33 Selection of the proper antimicrobials and
concentrations needed are critical for adequate product preservation, and understanding the
characteristics of the ideal preservative provides the basis for this.
THE IDEAL PRESERVATIVE
There has been an evolution of the characteristics of the “ideal preservative” that have been
identified over the past 75 years to incorporate both positive and negative characteristics,
including inactivating microorganisms quickly enough to prevent adaptation, no cross-
resistance with other preservatives or antibiotics, no negative environmental issues, no
adverse effects on the skin microbiome, and sustainability. The ideal preservative is one that
is both safe and effective for killing all types of microorganisms in all types of formulations,
but it has never been found.14 The reason for this is that no single preservative meets all the
characteristics of the ideal preservative, which are:
• It should have a broad spectrum of activity, which means that it would be effective
against all types of microorganisms so that it could be used as the only preservative in
a formulation.
• It should be effective at low concentrations and be active over the range of pH values
used in cosmetics and drugs.