387 The Human Stratum Corneum
of surfactants, with anionic surfactants showing higher irritation tendency compared to
amphoteric and nonionic surfactants. This is consistent with the pragmatic approach
that formulators have been using over the years to increase mildness towards skin by
increasing the size of the polar head group. For example, it is well known that harshness
of ethoxylated sulfates follow the order: SLS SLES 1EO SLES 2 EO SLES 3EO.
This can be generalized further with more quantitative relationships, relating headgroup
structural parameters to protein denaturation tendency.
The molecular mechanism involved in denaturation of proteins by surfactants in aqueous
solutions is thought to be due to the binding of surfactants to the proteins resulting in
the formation of micelle-like structures on the protein backbone and causing significant
electrostatic repulsion within the network.34 This in turn unravels the tertiary structure
of proteins. This charging of the structure in the case of insoluble proteins such as zein
results in dissolution of the protein. In contrast, in the case of crosslinked proteins such
as keratin, this results in osmotic driven swelling of proteins. Based on this hypothesis,
Lips et al. examined a correlation between micelle charge density and the dissolution of
zein using a variety of surfactants and found a linear correlation between micelle charge
density and the protein dissolution.34 Micelle zeta potential also correlated linearly with
the protein dissolution. Since the zeta potential of micelles is related to its charge density,
both the parameters correlating with the dissolution are not surprising, and overall, these
correlations support the hypothesis that micelle charge density is a predictive measure of
the denaturing potential, and hence the irritation tendency of the surfactants.
The role of micelle charge density as a key predictive parameter is further evident from the
work of Morris et al., which showed that the penetration of anionic surfactants into human
skin as measured from Franz diffusion experiments correlated linearly with the micelle
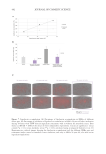
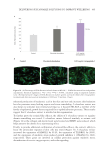
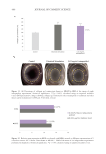
Figure 6. The correlation between cytokine release in-vitro and clinical TEWL measurement in a patch test.
Data from Walters et al. TEWL measurements are from in-vivo patch testing.45
of surfactants, with anionic surfactants showing higher irritation tendency compared to
amphoteric and nonionic surfactants. This is consistent with the pragmatic approach
that formulators have been using over the years to increase mildness towards skin by
increasing the size of the polar head group. For example, it is well known that harshness
of ethoxylated sulfates follow the order: SLS SLES 1EO SLES 2 EO SLES 3EO.
This can be generalized further with more quantitative relationships, relating headgroup
structural parameters to protein denaturation tendency.
The molecular mechanism involved in denaturation of proteins by surfactants in aqueous
solutions is thought to be due to the binding of surfactants to the proteins resulting in
the formation of micelle-like structures on the protein backbone and causing significant
electrostatic repulsion within the network.34 This in turn unravels the tertiary structure
of proteins. This charging of the structure in the case of insoluble proteins such as zein
results in dissolution of the protein. In contrast, in the case of crosslinked proteins such
as keratin, this results in osmotic driven swelling of proteins. Based on this hypothesis,
Lips et al. examined a correlation between micelle charge density and the dissolution of
zein using a variety of surfactants and found a linear correlation between micelle charge
density and the protein dissolution.34 Micelle zeta potential also correlated linearly with
the protein dissolution. Since the zeta potential of micelles is related to its charge density,
both the parameters correlating with the dissolution are not surprising, and overall, these
correlations support the hypothesis that micelle charge density is a predictive measure of
the denaturing potential, and hence the irritation tendency of the surfactants.
The role of micelle charge density as a key predictive parameter is further evident from the
work of Morris et al., which showed that the penetration of anionic surfactants into human
skin as measured from Franz diffusion experiments correlated linearly with the micelle
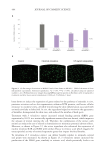
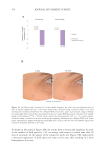
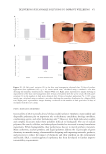
Figure 6. The correlation between cytokine release in-vitro and clinical TEWL measurement in a patch test.
Data from Walters et al. TEWL measurements are from in-vivo patch testing.45