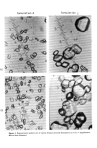
SWELLING OF STRATUM CORNEUM 529 Table VII Effect of Sodium Laurate (NaL) on Work Index of Stratum Corneum Standard Treatment Work Index Deviation Water 1 per cent sodium acetate, pH 9.8, 60 rain 1 per cent NaL, pH 9.8, 60 min 0.98 -+0.07 7 0.88 -+0.07 5 0.50 -+0.17 7 aNumber of measurements. 3O 20 10 i I '/0 0 0 0 I ß NaLS o NaL ß o 0 I I i i 0 5 10 20 24 Time [hrs.] Figure 2. Rate of in-plane swelling in sodium lauryl sulfate (NaLS) and sodium laurate (NaL) ment produced weakened stratum corneum. The data in Table VII indicate that water and sodium acetate had little effect on the strength of the tissue while sodium laurate produced a notable weakening. Rate studies of stratum comeurn swelling in sodium laurate and sodium lauryl sulfate (Fig. 2) justified the brief exposure to surfactant em- ployed in the mechanical test. DISCUSSION The use of in-plane swelling of guinea pig stratum corneum is an admittedly insensitive means of determining surfactant-skin interactions. Nevertheless, the procedure is simple, requires little time by an experimenter and no sophisticated equipment. That the values obtained for a given surfactant can differ with the source of the stratum cor- neum (animal to animal) and even with the site on an animal (piece to piece) is evident from the high standard deviations. Thus, in comparing surfactants, stratum corneum
530 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS from the same animal and from neighboring sites should be used. Fortunately, we have observed that although the magnitude may be variable from study to study, the relative order of magnitude remains the same and surfactants can easily be classified as produc- ing little or no swelling, or producing readily measurable swelling. Scheuplein and Ross (4) soaked human stratum corneum in 5 per cent sodium laurate for up to 72 h and ob- served an expansion in area ranging from 50 to 80 per cent. In discussing the mechanism by which anionic detergents swell stratum corneum while cationic and nonionic surfactants appear to have little effect, it seems reasonable to briefly consider the literature on globular proteins. As summarized by Tanford (9) am- phiphilic substances (ionic or polar derivatives of hydrocarbons) can combine with pro- teins in at least 3 distinct modes of interaction. (1) Association with specific binding sites of native proteins (serum albumin and fi-lactoglobulin behave in this manner) (2) Cooperative association between protein and a large number of detergent molecules without major conformational changes (serum albumin with alkyl sulfates and sulfo- nates having short hydrocarbon chains) (3) Cooperative association with gross denatu- ration of the protein. The 3 types of binding occur with detergent monomers rather than micelies. In fact, micelie formation can be considered to be in competition with protein binding. Nevertheless, to insure a maximum concentration of monomer, our studies were performed well above the CMC except where noted. Since nonionic detergents have much lower CMC than anionic detergents, fewer monomers would be present in a solution of nonionic detergent so that binding type 3 would be less likely to occur with a nonionic detergent (10). It should be noted that lit- tle or no swelling was observed with nonionic detergents. Tanford and coworkers (11) also compared the interaction of anionic lauryl sulfate and tetradecyltrimethylammonium ions with serum albumin and other globular proteins and concluded that both detergents yield type 3 interaction. However, with the cat- ionic detergent type 3 binding occurred very close to its CMC while with lauryl sulfate the binding occurred far below its CMC. Less cationic detergent was bound than anionic detergent. The authors concluded that the difference between the 2 types of detergent was that the anionic detergent would cluster around the longer cationic sites in the protein (arginyl and lysyl side chains) which could accommodate more detergent molecules than the shorter anionic sites in the protein (glutamyl, aspartyl) around which cationic detergents would cluster. In a subsequent paper, however, Tanford and coworkers (12) studied the binding of the same cationic detergent as well as lauryl sulfate to apoproteins of human serum high density lipoprotein and observed coopera- tive interaction with the cationic detergent at a much lower equilibrium detergent concentration than observed previously with water-soluble proteins. They also con- cluded that binding the cationic and anionic detergent had resulted in a change in con- formation which was unlike the denatured state observed with globular proteins. That little swelling was observed with the cationic surfactants we used might be due to the low CMC for Triton X-400, or it might reflect the inability of the stratum corneum to undergo cooperative binding with these particular surfactants. Preliminary work sug- gests that dodecyltrimethylammonium chloride may produce levels of swelling similar to lauryl sulfate. The results of the 2 studies just cited indicate the difficulty in choosing the appropriate soluble protein to simulate the insoluble stratum corneum. However, as the interests of academic scientists turn more toward membranous proteins, more choices for the cosmetic chemist/skin biologist should be available.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)