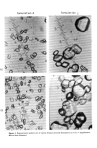
Formulation A Formulation C •. •--•k• ' "•-'-.•- •&•" •'"'• ............ • • . Figure 2. Representative particle size of various benzoyl peroxide formulations at 312.5 x magnification. Micron Scale illustrated.
546 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS Table X Particle Size of Various BPO Formulations Per Cent Found in Different Formulations Particle Size (/x) A C 20 90 50 20, 40 10 40 40 1 10 upon this acute oral toxicity study in mice, benzoyl peroxide 10 per cent is considered not to represent an acute oral hazard should accidental oral ingestion occur. Topical ocular instillation ofbenzoyl peroxide 10 per cent (A) and marketed competitor products (C, D, and B) induced substantial ocular changes characterized by severe con- junctival congestion, swelling, discharge, minimal iritis and flare, moderate to severe corneal cloudiness, and evidence ofpannus. These ocular changes are interpreted to be indicative of a test formulation which has a marked ocular irritation potential. Based upon these studies, it is concluded that precautionary labeling should accompany all benzoyl peroxide 10 per cent formulations. It is recommended that a precautionary statement state, "avoid contact with eyes and mucous membranes." In the dermal irritation studies, 1 mortality occurred with formulation C and vehicle groups. It is not considered to be treatment related but related to the handling of the animals. Observed dermal irritation was minimal to none for all formulations. Benzoyl peroxide 10 per cent A and C, D, and B induced comparable minimal dermal erythema during the 3-day derreal test, which is substantially less than that for the posi- tive control. The positive control test formulation has been reported to cause dermal erythema and edema in experimental animals (9). This amount of dermal erythema ob- served for the benzoyl peroxide formulation is considered to be indicative of a low dermal irritation potential. Based on this rabbit study, it is concluded that benzoyl peroxide 10 per cent A has a low dermal irritation potential, equivalent to that for marketed competitor products, C, D, and B. It is also concluded that all benzoyl peroxide 10 per cent formulations do not represent an acute dermal hazard for intended clinical use. The particle size relationships observed could be linked to observed increased ocular irritation. Particle sizes for benzoyl peroxide A, D, and B were of a lesser magnitude than for formulation C (Table X, Fig. 2). In a clinical test, C was much more irritating in 24-h occlusive patch test. Surprisingly, one formulation D appeared minimally irritating (Grade 1) of all formulations initially, but produced a delayed response 48 h after removal of the patch. The significance of this response can be related to vehicle differences and is still under investigation. The effect of benzoyl peroxide formulations on cutaneous bacteria has been reported to be most significant on anaerobes, which comprise about 98 per cent of Corynebacte- rium acnes (10). This reduction of anaerobes appear to be unrelated to free fatty acid (Table XI). Vehicles play an important part of optimizing the antibacterial properties
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)