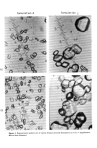
BENZOYL PEROXIDE FORMULATIONS Table VI Iritis for Rabbits After Single Topical Ocular Instillation Of Benzoyl Peroxide 10 Per Cent 541 Test Formulation Iritis Wash Time c 1 h 24 h 48 h 72 h 7 day 14 day 21 day Day 0 Controls Day 0 Day 1 Controls Day 1 Untreated Controls -- 20sec •a 0.0 0.5 0.0 0.0 0.0 0.0 ] 0.0 Inc? 0/6 2/6 0/6 0/6 0/6 0/6 0/6 5min • 0.0 0.7 0.5 0.3 0.0 0.0 0.0 Inc. 0/6 3/6 2/6 2/6 0/6 0/4 d 0/4 24h • 0.0 0.5 0.2 0.2 0.3 0.0 0.0 Inc. 0/6 2/6 1/5 1/5 1/4 0/6 0/6 20sec • 0.7 1.5 1.7 0.3 0.5 0.0 0.0 Inc. 4/6 5/6 4/6 2/6 1/4 0/6 0/6 5 min • 0.2 1.0 1.8 1.8 0.0 0.0 0.0 Inc. 1/6 3/6 4/5 4/4 0/3 0/3 0/3 24h • 0.2 0.7 1.3 1.8 0.5 0.0 0.0 Inc. 1/6 3/6 4/6 4/4 1/2 0/2 0/3 • 0.0 0.0 0.0 0.0 0.0 0.0 0.0 Inc. 0/6 0/6 0/6 0/6 0/6 0/6 0/6 • 0.0 0.0 0.0 0.0 0.0 0.0 0.0 Inc. 0/6 0/6 0/6 0/6 0/6 0/6 0/6 • 0.0 0.0 0.0 0.0 0.0 0.0 0.0 Inc. 0/6 0/6 0/6 0/6 0/6 0/6 0/6 aMean score calculated by summing individual scores and dividing score: iritis = 4. bNumber of eyes with response/number of eyes in test group. ½Interval of time after dosing until eye was washed. dNumber less than 6 indicates mortality not related to treatment. by the number of observations. Maximum Relative to iritis, 2 instances of minimal iritis was observed at the 24-h observation pe- riod for the 20-sec treatment group for eyes receiving the benzoyl peroxide formula- tions. Minimal iritis at a low incidence was observed for the 24-, 48-, and 72-h periods for the 5-min wash group. Iritis was not observed for the remainder of the observation periods for these two groups. Minimal iritis at a low incidence was observed at 24, 48, and 72 h on Day 7 for the 24-h wash group for eyes which received benzoyl peroxide formulations. Iritis was not observed for any other observation period. Minimal iritis at a low to moderate incidence was observed at 1, 24, 48, and 72 h and again on Day 7 for the 20-sec and 24-h wash groups for eyes which received some of the benzoyl peroxide formulations. Iritis was not observed on Days 14 or 21. Minimal iritis at a low to high incidence was observed at 1, 24, 48, and 72 h for eyes for the 5-min wash group for eyes which received some benzoyl peroxide formulations iritis was not observed on Days 7, 14, and 21. Iritis was not observed for any control eyes (Table VI). Minimal iritis was observed in formulation C with none observed with formulation A. With regard to severity of corneal cloudiness, a low to high incidence was observed on the 1-, 24-, and 48-h observation periods for the 20-sec wash group for eyes which received various benzoyl peroxide formulations thereafter, corneal cloudiness was not observed for any eye in this treatment group. The area (of involvement relative to the
542 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS Table VII Severity of Corneal Cloudiness for Rabbits After Single Topical Ocular Instillation of Benzoyl Peroxide 10 Per Cent Test Wash Severity of Corneal Cloudiness Formulation Time" 1 h 24 h 48 h 72 h 7 day 14 day 21 day A 20sec A 5 min A 24 h C 20 sec C 5 min C 24h Day 0 Controls Day 0 Day 1 Controls Day 1 Untreated Controls -- Inc? Inc. Inc. Inc. Inc. Inc. Inc. Inc. Inc. 0.7 1.2 0.3 0.0 0.0 0.0 0.0 4/6 5/6 2/6 0/6 0/6 0/6 0/6 1.3 2.3 2.3 2.3 1.0 0.2 0.3 6/6 6/6 6/6 6/6 5/6 1/4 d 1/4 1.0 1.5 1.7 1.7 1.8 0.7 1.0 5/6 5/6 4/6 4/6 4/6 4/6 3/6 1.0 1.7 2.2 2.0 2.2 0.7 0.5 I 6/6 6/6 6/6 5/6 5/6 4/6 3/6 1.0 1.8 2.7 3.2 3.5 2.5 3.0 6/6 6/6 6/6 6/6 6/6 6/6 6/6 1.0 2.0 2.3 3.2 3.3 2.8 2.3 6/6 6/6 6/6 6/6 5/6 5/6 4/6 0.0 0.0 0.0 0.0 0.0 0.0 0.0 0/6 0/6 0/6 0/6 0/6 0/6 0/6 0.0 0.0 0.0 0.0 0.0 0.0 0.0 0/6 0/6 0/6 0/6 0/6 0/6 0/6 0.0 0.0 0.0 0.0 0.0 0.0 0.0 0/6 0/6 0/6 0/6 0/6 0/6 0/6 aMean score calculated by summing individual scores and dividing scores: corneal cloudiness = 4. •Number of eyes with response/number of eyes in test group. qnterval of time after dosing until eye was washed. dNumber less than 6 indicates mortality not related to treatment. by the number of observations. Maximum total surface of cornea) of corneal cloudiness was approximately 25 to 50 per cent. Minimal corneal cloudiness at a moderate to high incidence was observed at 1, 24, 48, and 72 h and again on Day 7 for the 5-min and 24-h wash groups for the eyes which received various benzoyl peroxide formulations. On Days 14 and 21, minimal corneal cloudiness at a low to moderate incidence was observed for the same treatment groups (Table VII). Area for corneal cloudiness for these treatment groups on Day 7 was 50 to 100 per cent and on Day 14 approximately 24 per cent. Minimal to moderate corneal cloudiness at a high incidence was observed at 1, 24, 48, and 72 h and again on Days 7 and 14 for the 20-sec, 5-min, and 24-h wash groups for eyes which received some of the benzoyl peroxide formulations. On Day 21, minimal corneal cloudiness at a moderate incidence was observed for the 20-sec wash group, while moderate corneal cloudiness at a moderate to high incidence was observed for the 5-min and 24-h wash groups for eyes which received benzoyl peroxide formulations. The corneal area through Day 14 for each of the 3 treatment groups was approximately 75 to 100 per cent, while on Day 21 it was approximately 25 to 50 per cent. Corneal cloudiness was not observed for any control group (Table VIII). A greater severity of corneal in- volvement was noted for formulation C than for formulation A at the 20-sec period.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)