BENZOYL PEROXIDE FORMULATIONS Table VIII Area of Corneal Cloudiness for Rabbits After Single Topical Ocular Instillation of Benzoyl Peroxide 10 Per Cent 543 Test Wash Formulation Time •' Area of Corneal Cloudiness lh 24h 48h 72h 7day 14day 1.5 2.0 0.3 0.0 0.0 0.0 0.0 4/6 5/6 2/6 0/6 0/6 0/6 0/6 3.8 2.3 0.3 0.3 6/6 5/6 1/4 d 1/4 2.7 2.0 1.7 0.8 4/6 4/6 4/6 3/6 21 day A 20 sec •a Inc. b A 5 min • Inc. _ A 24 h x Inc. C 20sec • Inc. C 5 min • Inc. C 24h • Inc. Day 0 Controls Day 0 • Inc. Day 1 Controls Day 1 • Inc. Untreated Controls -- • Inc. 4.O 4.O 3.8 6/6 6/6 6/6 2.7 2.7 2.7 5/6 5/6 4/6 3.2 3.8 3.3 3.0 2.3 1.8 1.3 6/6 6/6 6/6 5/6 5/6 4/6 3/6 3.8 3.8 4.0 4.0 3.5 3.5 2.7 6/6 6/6 6/6 6/6 6/6 6/6 6/6 4.0 4.0 4.0 4.0 3.2 3.2 2.5 6.6 6.6 6.6 6.6 5/6 5/6 4/6 0.0 0.0 0.0 0.0 0.0 0.0 0.0 O/6 O/6 O/6 O/6 O/6 O/6 O/6 0.0 0.0 0.0 0.0 0.0 0.0 0.0 O/6 O/6 O/6 O/6 O/6 O/6 O/6 0.0 0.0 0.0 0.0 0.0 0.0 0.0 O/6 O/6 O/6 O/6 O/6 O/6 O/6 aMean score calculated by summing individual scores and dividing scores: corneal cloudiness = 4. bNumber of eyes with response/number of eyes in test group. qnterval of time after dosing until eye was washed. •Number less than 6 indicates mortality not related to treatment. by the number of observations. Maximum Infrequent instances of minimal intensity offluorescein staining occupying less than 25 per cent of the corneal surface area was observed for eyes which received all test formulations, while fluorescein staining was not observed for any control eye. Relative to pannus, in the 24-h wash group which received benzoyl peroxide formula- tion, pannus at a moderate incidence was observed on Days 7, 14, and 21. Pannus was not observed for the 20-sec or 5-rain wash groups for eyes which received benzoyl peroxide formulations "A" at 10 per cent. Moderate to marked pannus was observed on Days 7, 14, and 21 for the 5-rain and 24-h wash groups for eyes which received benzoyl peroxide formulations "C." Pannus was not observed for any control eyes (Ta- ble IX). Pannus for formulation C was more severe than for A. DERMAL IRRITATION Minimal to moderate dermal edema and erythema at a high incidence was observed for the rabbits which received the positive control test formulation on each of the 3 test days. Dermal edema and erythema was not observed for the test sites receiving any of the benzoyl peroxide formulations or for the untreated control sites.
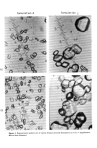
544 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS Table IX Pannus for Rabbits After Single Topical Ocular Instillation of Benzoyl Peroxide 10 Per Cent Test Wash Formulation Time c 1 h 24 h 48 h 72 h 7 day 14 day 21 day A 20 sec 0.0 0.0 0.0 0/6 0/6 0/6 A 5 min A 24h C 20 sec C 5 min C 24h Day 0 Controls Day 0 Day I Controls Day 1 Untreated Controls -- •a 0.0 0.0 0.0 0.0 Inc? 0/6 0/6 0/6 0/6 • o.o o.o o.o o.o Inc. if6 0/6 0/6 0/6 • o.o o.o o.o o.o Inc. 0/6 0/6 0/6 0/6 • o.o o.o o.o o.o Inc. 0/6 •6 0/6 0/6 • 0.0 0.0 0.0 0.0 Inc. 0/6 0/6 0/6 0/6 • o.o o.o o.o o.o Inc. 0/6 •6 0/6 0/6 • o.o o.o o.o o.o Inc. 0/6 0/6 0/6 0/6 • 0.0 0.0 0.0 0.0 Inc. 0/6 0/6 0/6 0/6 • 0.0 0.0 0.0 0.0 Inc. 0/6 0/6 0/6 0/6 0.0 0.0 0.0 0/6 0/4 a 0/4 0.7 1.0 0.7 2/6 3/6 2/6 1.0 1.0 0.0 3/6 4/6 0/6 1.8 1.8 1.7 6/6 6/6 5/6 1.7 1.5 1.3 5/6 5/6 4/6 0.0 0.0 0.0 0/6 0/6 0/6 0.0 0.0 0.0 0/6 0/6 0/6 0.0 0.0 0.0 0/6 0/6 0/6 aMaximum score = 2. bNumber of eyes with response/number of eyes in test group. Clnterval of time after dosing until eye was washed. aSacrificed in moribund condition on day 7. PARTICLE SIZE DETERMINATION Figure 2 demonstrates the various particle sizes which are observed under the Zeiss microscope. The range is shown in Table X. Particle size distribution varied between less than 20/x to greater than 40/x. A comparative study of particle size to a patch test undertaken in our clinic demonstrated that the larger particle size correlated with the greater amount of immediate type of irritation observed in Formulation C. However, some vehicle effects determined a late type of irritation, which will be described in the discussion. DISCUSSION The acute oral LD50 in mice for benzoyl peroxide 10 per cent (A) was 14 ml/kg. This oral LD.50 is statistically equivalent to that of competitor products, C, D, and B, whose oral LD.•0 was 12, 10, and 12 ml/kg, respectively. Based upon the scale of Spector (8) and converting g/kg to ml/kg, benzoyl peroxide 10 per cent formulation has a rating of practically nontoxic. For an average 6-month-old 10 kg child, approximately 140 ml would have to be ingested before approaching the oral LD50 in this mouse study. For the average 70 kg adult, approximately 980 ml of benzoyl peroxide 10 per cent would have to be orally ingested before approaching the oral LDs0 in this mouse study. Based
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)