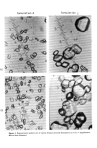
Jo Soc. Cosmet. Chem., 28, 533-549 (September 1977) Some comparisons of benzoyl peroxide formulations O. J. LORENZETTI, T. WERNET, and T. McDONALD Alcon Laboratories, Inc., 620i South Freeway, Fort Worth, TX 76101. Received November 17, 1976. Presented Ninth IFSCC Congress, June i976, Boston, MA. Synopsis The BIOLOGICAL PROFILE of several FORMULATIONS of BENZOYL PEROXIDE are compared. The efficacy of benzoyl peroxide can be optimized by influencing formulation variables. A benzoyl peroxide formulation "A" is compared to some competitive products, C, D, and B. Derreal irritation in rabbits, acute toxicity in rats, efficacy against Corynebacterium acnes, as well as several formulation variables, such as particle size and vehicle interaction, are compared. The selection of a candidate formulation is subjected to test involving acute toxicity, ocular irritation, skin ir- ritation, and sensitization. The design of these tests should involve not only positive and negative controls, but also appropriate reference products which are already on the market. It is important when developing biological data to accumulate data on the finished product, since it is the finished product which will come into extensive contact with the skin in a clinical population. All the formulations tested were equivalent in performance except for ocular irritation potential. Formula- tions with larger particle size distribution had a greater ocular irritation potential. INTRODUCTION Benzoyl peroxide is an old and established treatment agent with keratolytic and an- tibacterial action. This compound is formed by the reaction of alkaline (sodium) hy- drogen peroxide with benzoyl chloride in water. It was described as a therapeutic aid in the treatment of burns, ulcerations, and various infected cutaneous and mucous membrane lesions, and more recently it has been found to be among the most effective mode of acne therapy (1). There are many methods to treat ache vulgaris, including unblocking the piloseo baceous duct, strengthening the wall of the duct, decreasing the amount of sebum secreted, or changing the composition of the sebum to make it less of an irritant (2). Among the most popular method of treatment of acne is the use of benzoyl peroxide. The topical use of drying and peeling medications has long been a mainstay of the therapy of acne vulgaris. It is now fairly well agreed that the initial local changes in ache consist of desquamation and hyperkeratosis of the follicular orifice of the functioning sebaceous gland, thus producing an obstruction and dilation of the glandular opening with retention of the sebum. The effect of these peeling agents is obvious. The most 533
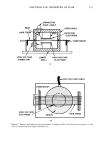
534 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS STRUCTURE 0 0 II tl USE - A desquamating antimicrobial agent used in treatment of acne. MECHANISM OF ACTION - - slow oxygen releaser providing antimicrobial activity - scaling (comedolytic) - reduction of free fatty acids (FFA) released during metabolism of surface bacteria Figure 1. What is benzoyl peroxide? direct way to interrupt this process that leads to the formation of ache lesions is by peeling away of the rim of the hyperkeratotic orifice. Many agents have been utilized to this end, some more successfully than others. The purpose of this paper is to review the profile of several benzoyl peroxide formula- tions, which have been investigated in our laboratories. Evaluations were based on studies undertaken on acute toxicity, ocular irritation, derreal irritation, as well as several formulation variables as particle size and vehicle interaction (3, 4) (Fig. 1). For brevity, data on two formulations A and C will be presented at 10 per cent concentration of benzoyl peroxide. These were selected because they represented the 2 extremes in formulation response variables and the data is representative. Formula- tions D and B gave response intermediate between A and C. MATERIALS AND METHODS Several standard methodologies were used for these studies and will be briefly men- tioned. ACUTE ORAL TOXICITY Swiss albino mice weighing approximately 20 g• were utilized. The mice were accli- mated to laboratory conditions for 1 week prior to use. The animals were maintained on Purina Chow-• and tap water provided ad libitum. The test formulation was appro- *Obtained from Mouse House, Marlow OK. q-Ralston Purina.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)