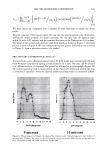
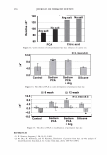
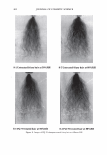
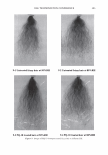
2006 TRI/PRINCETON CONFERENCE 321 cut into 2-3 mm lengths and conditioned overnight. The next day 10-12 mg of hair was placed under climate controlled conditions into a medium pressure 120 µl volume, steel crucible (Netzsch) with a lid and sealing elastomer, max pressure 20 bar max temperature 200°C. 50 µl of distilled water was added to the crucible which was then sealed. 4 replicates are used for each determination. • A Dynamic Difference Calorimeter DSC7 calibrated with indium supplied by Perkin Elmer. The heating range was 70-170°C at a rate of 10°C I minute. Hair tresses were conditioned for 24 hours at RH 55% and 22°C before samples were removed and cut into 0.5mm lengths. 4-7mg of hair was placed into a Perkin Elmer pan. 50µ1 of distilled water was added to the crucible which is then sealed. At least 3 replicates were used for each determination. The tensile properties of the fibres were measured using a Diastron Miniature tensile tester (MTT 675) equipped with laser micrometer. The color of the hair was measured with a bench top Minolta CM3600D spectropho- tometer. Lab values were calculated under D65 illuminant, 10° observer, specular in- cluded. The lightening (dL) was calculated as the difference in L value between the final color and the starting color on the untreated hair. RES UL TS AND DISCUSSION The results detail the effect on the HPDSC denaturation temperature and enthalpy following treatments with commercial retail bleach products A and B. Both products have three components: the alkalizer, the hydrogen peroxide, and the persulfate salt powder. These two products have the same level of oxidant (hydrogen peroxide and ammonium persulfate) and the formulations of these two components are very similar. However the formulations of the alkalizer components are different. Product A has a gel alkalizer formulation with ethanolamine as the alkalizer Product B has a liquid alkalizer formulation with ammonium hydroxide as the alkalizer. Three repeat treatments were performed with five wash cycles in between each treat- ment. After this treatment protocol the swatches were analysed for lightness vs. the starting substrate (dL), tensile strength and HPDSC peak temperature and enthalpy. Table I summarises the results of these analyses. For the HPDSC determination, the results show that both of the bleach products induce a decrease in peak temperature vs. the untreated hair as one would expect from previ- Table I The Effect of Treatments of Bleach Formulations on HPDSC and Tensile Strength Tensile strength measurements Plateau load Load@ 25% Break load Peak temp. Enthalpy dL after 3 Gmf/ sq .micron Gmf/sq.micron Gmf/sq.micron TD ± s LlHD ± s Product cycles (x 103) ( X 103) (x 103) (oC) 0/g) Untreated hair 0 6.47 ± 0.23 7.52 ± 0.23 20.9 ± 1.9 148.3 ± 0.1 6.7 ± 0.5 Bleach A 48 ± 0.6 4.00 ± 0.27 4.81 ± 0.34 16.5 ± 1.5 143.8 ± 0.8 5.8 ± 0.5 Bleach B 49 ± 0.5 4.12 ± 0.38 4.70 ± 0.45 16.5 ± 3.7 138.8 ± 0.3 5.1 ± 0.3
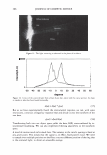
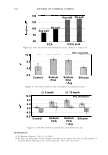
322 JOURNAL OF COSMETIC SCIENCE ously published work. However, there is a significant difference of 5 .1 °C in denaturation peak temperature between the two products. Further, for the treated switches, there is no significant difference between the lightening (dl) values and the tensile parameters which are also indicators of oxidative covalent bond cleavage. In addition, the amount of oxidant in the systems is the same so the proposed explanation for this data was that there were other factors that were contributing to the decrease in peak temperature. It was hypothesized that these two sets of results as described above are due, in part, to the incorporation of different actives into the fibre such as salts, alkalizers and formu- lation actives during the treatment process. For these two products tested, the formu- lations were different and contain different alkalizers, salts and formulation actives. A possible mechanism is that these product components are able to penetrate inside the fibre and change the stability and viscosity of the IFAP proteins by changing the arrangement of the electrostatic and hydrogen bonds. These changes could affect the immediate environment of the intermediate filaments and in turn their denaturation temperature and enthalpy. To test this hypothesis we would predict that either on dialysis of the hair or repeated washing cycle, these components would slowly diffuse out of the hair and the peak temperature would increase back toward the untreated hair values. This means that the changes in peak temperature would be reversible. The starting untreated hair was treated with just one component of the colorant and bleach formulations the alkalizer. All bleach products contain an alkalizer to ensure the product is at the pH required for effective lightening and in the majority of colorant and bleach products this alkalizer is either ammonia, ethanolamine or silicate. Two swatches of untreated hair were soaked in the alkalizer (1.27M) for 30 minutes, the same time as the bleach treatment. The swatches were then rinsed in tap water for one minute and then treated with one wash cycle (i.e. 2 shampoos). The HPDSC peak temperature and denaturation enthalpies were measured both after treatment and after dialysis of the hair in deionised water. To perform the dialysis the hair was first soaked in 50ml of deionised water followed by 100ml of deionised water followed by 11 of deionised water. The pH was measured at all three stages. The hair was then soaked in 201 of deionised water over a 24 hour period where the water was replenished six times (i.e. swatch exposed to 1201 of deionised water). The tensile strength of the hair was also measured before dialysis. There was no expected or observed lightening so dl is not reported. Table II summarises the results of the HPDSC and tensile strength. Table III summarises the pH measurements on dialysis for the ammonium hydroxide alkalizer. The results support the hypothesis that selected components of the bleach products can lower the denaturation peak temperature and enthalpy and that this effect is at least partially reversible on dialysis. The lightening and tensile strength data confirm that the drop in HPDSC peak temperature and enthalpy is not due to oxidative covalent bond cleavage as expected as we have no oxidant present. In addition, the pH data indicates that even after rinsing and two shampoos there is still residual alkalinity in the hair that is only gradually removed by the dialysis. It is hypothesised that the residual alkalinity is participating in electrostatic and hydrogen bonding interactions with the IFAPs which will change the viscosity of the matrix and cause a change in the HPDSC peak
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)